Annual Report 2020
Department of Breast and Medical Oncology
Kan Yonemori, Tatsunori Shimoi, Kazuki Sudo, Emi Noguchi, Maki Tanioka, Tadaaki Nishikawa, Toshihiro Okuya, Yuki Kojima, Hitomi Sumiyoshi-Okuma, Shu Yazaki, Yohei Chiba, Yasuhiro Fujiwara
Introduction
The Department of Medical Oncology provides the most effective treatments by the use of chemotherapy, and works on the establishment of new standards of care for adult malignancies including breast cancer, gynecologic cancer, soft-tissue sarcoma, extra-gonadal germ cell tumor, cancer of unknown primary and other rare types of solid tumors.
We envision becoming a leading medical oncology department, which makes a difference in cancer care in Japan and throughout the world. Our mission is to provide patient-centered, state-of-the-art medical care to cancer patients, to develop new effective cancer treatments through clinical and translational research, and to nurture medical oncologists. An evidence-based, research-oriented and multidisciplinary approach is the core value of our practice.
The Team and What We Do
1. Setup
Our division consists of seven full-time attending physicians, two physicians with concurrent appointments in research departments, three to four chief residents (fellows), and two - to three clinical residents. We also provide educational opportunities to short-term (a half -year) residents. Full-time attending physicians are on duty at the outpatient clinic one to two days per week. Inpatient management is undertaken by clinical teams, which consist of attending physicians and residents. A "Grand Round" is scheduled every Monday.
2. Performance
There were 1625 first visits of new patients in 2020 (Table 1). Of these visits, 33% were cases of breast cancer; 19%, gynecologic cancer; 14%, cancer of unknown primary; and 16%, soft-tissue sarcoma. We also provided 544 second opinions in 2020.
Table 1. 1st visit patients to the Department of Medical Oncology (Apr. 2019 - Mar. 2020)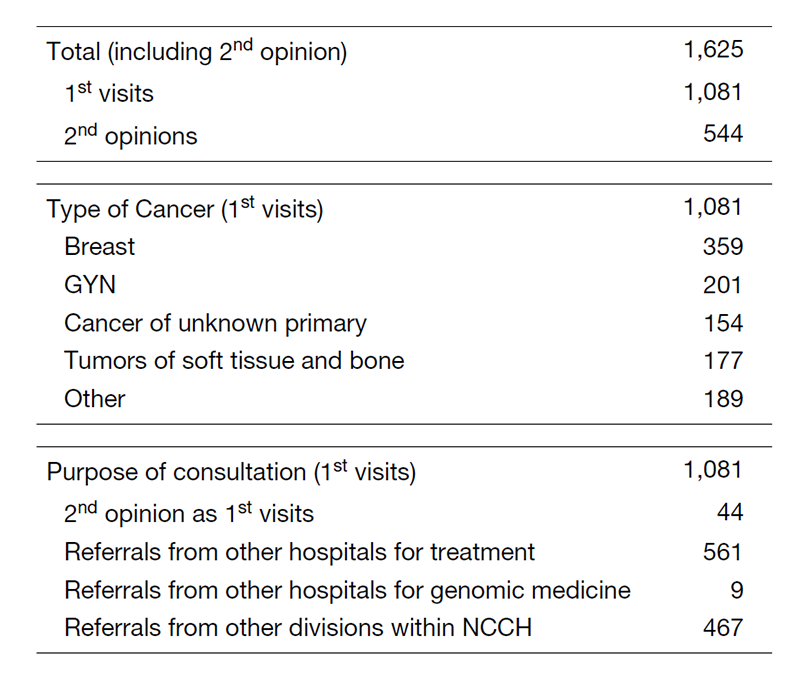

3. Conferences
One-hour briefing medical conferences are held every morning to discuss the evidence-based care for individual patients. The Phase 1 conference is held on Mondays, Journal Club on Wednesdays, Clinical trial conference as well as the Weekend and Outpatient follow-up conference on Fridays. Multidisciplinary case conferences with diagnostic radiologists, surgeons, and pathologists are held with members of the departments of Breast Surgery, Gynecology, Musculoskeletal Oncology and Rehabilitation, Radiation Oncology and Pathology, each once or twice (breast) per week.
The Monthly Breast Cancer Conference is held with the participation of the multidisciplinary specialists to discuss recent topics on breast oncology and to update institutional treatment guidelines. This year, we published “Nyugan-Shinnryou Application Notebook” from Nankodo based on this these guidelines. This document reflects the consensus of the breast cancer team on the body of evidence addressing breast cancer management.
Research activities
Our research interests extend across a wide range of topics related to treatment and clinical program development. Many of our research programs are secured by public and consignment research grants. In 2020, we conducted many research programs as a primary investigator and participated in additional programs as a co-investigator in research programs secured by competitive public research funds. We published 44 international manuscripts, focusing on early-phase anti-cancer drug development, molecular imaging, drug efficacy studies using patient-derived xenografts, translational research, novel chemotherapy against sarcoma and ovarian cancer, novel biomarkers to predict efficacy and adverse events of anti-cancer drugs and other basic research. We value cancer survivorship as a research theme to develop a comprehensive patient-centered care program.
Clinical trials
In 2020, we actively enrolled patients in Phase I studies (including first-in-human or global) as well as domestic and international Phase II and III studies (Table 2). Of note, we enrolled patients in investigator-initiated clinical trial (IIT) for rare cancers. For example, we enrolled them in DS8201, monotherapy in carcinosarcoma patients positive for HER2 in rare tumor patients of all types, as an IIT (Table 2). We also conducted many types of translational research (TR) to find novel biomarkers.
Education
We provide rich educational opportunities to both residents and chief residents through clinical experience as well as research activities. Residents are encouraged to make presentations at both local and national conferences. We vigorously support basic, clinical, or translational research conducted by postdoctoral researchers.
Future Prospects
We will continue to establish new standard treatments and propose a near-future model of clinical management of adult solid tumors, including breast cancer and gynecologic cancer. Moreover, we aim to build a comprehensive program, which includes tumor registry, translational research, clinical trials and patient care in rare adult tumors based on our rich clinical experience. We also strive to improve the efficiency of anti-cancer drug development by coordinating basic and translational research in early-phase clinical trials.
Table 2. Active clinical trials (Apr. 2020 - Mar. 2021)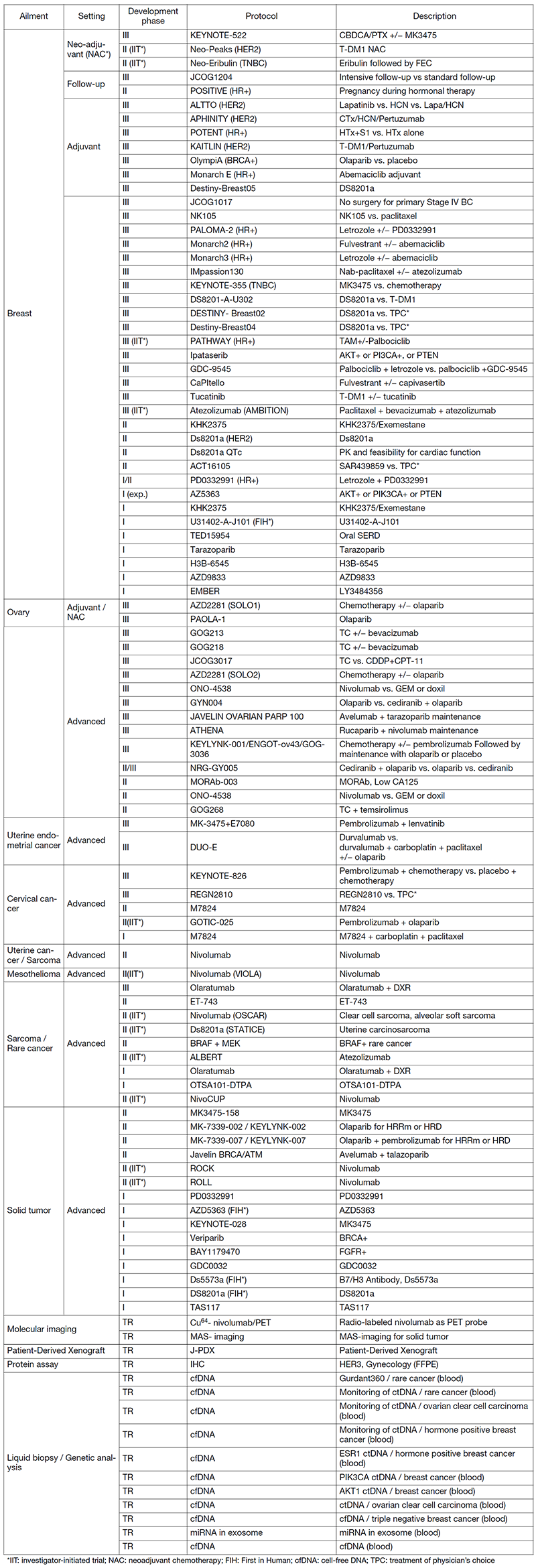

List of papers published in 2020
Journal
1. Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, Kakishima H, Yoshida H, Hishiki T, Kawakubo N, Kuroda T, Kiyokawa T, Yamada K, Yanaihara N, Takahashi K, Okamoto A, Hirabayashi S, Hasegawa D, Manabe A, Ono K, Matsuoka M, Arai Y, Togashi Y, Shibata T, Nishikawa H, Aoki K, Yamamoto N, Kohno T, Ogawa C. Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants. N Engl J Med, 384:42-50, 2021
2. Bun S, Yonemori K, Sunadoi H, Nishigaki R, Noguchi E, Okusaka T, Nishida T, Fujiwara Y. Safety and Evidence of Off-Label Use of Approved Drugs at the National Cancer Center Hospital in Japan. JCO Oncol Pract, 17:e416-e425, 2021
3. Hata T, Nakamura K, Yonemori K, Noguchi E, Watanabe M, Sohn J, Lu YS, Yap YS, Tamura K, Fujiwara Y. Regulatory and operational challenges in conducting Asian International Academic Trial for expanding the indications of cancer drugs. Clin Transl Sci, 14:1015-1025, 2021
4. Kitadai R, Shimoi T, Sudo K, Noguchi E, Nagata Y, Sawada R, Takashima A, Boku N, Yonemori K. Efficacy of second-line treatment and prognostic factors in patients with advanced malignant peritoneal mesothelioma: a retrospective study. BMC Cancer, 21:294, 2021
5. Nakamura IT, Ikegami M, Hasegawa N, Hayashi T, Ueno T, Kawazu M, Yagishita S, Goto Y, Shinno Y, Kojima Y, Takamochi K, Takahashi F, Takahashi K, Mano H, Kohsaka S. Development of an optimal protocol for molecular profiling of tumor cells in pleural effusions at single-cell level. Cancer Sci, 112:2006-2019, 2021
6. Noguchi E, Shien T, Iwata H. Current status of PD-1/PD-L1 blockade immunotherapy in breast cancer. Jpn J Clin Oncol, 51:321-332, 2021
7. Okagawa Y, Yoshinaga S, Noguchi E, Sekine S. Gastric metastasis from primary leiomyosarcoma of the broad ligament. Jpn J Clin Oncol, 51:846-847, 2021
8. Sagara Y, Mori M, Yamamoto S, Eguchi K, Iwatani T, Naito Y, Kogawa T, Tanaka K, Kotani H, Yasojima H, Ozaki Y, Noguchi E, Miyasita M, Kondo N, Niikura N, Toi M, Shien T, Iwata H. Current Status of Advance Care Planning and End-of-life Communication for Patients with Advanced and Metastatic Breast Cancer. Oncologist, 26:e686-e693, 2021
9. Pascual T, Fernandez-Martinez A, Tanioka M, Dieci MV, Pernas S, Gavila J, Guarnieri V, Cortes J, Villagrasa P, Chic N, Vidal M, Adamo B, Muñoz M, Griguolo G, Llombart A, Conte P, Oliveira M, Conte B, Paré L, Galvan P, Carey LA, Perou CM, Prat A. Independent Validation of the PAM50-Based Chemo-Endocrine Score (CES) in Hormone Receptor-Positive HER2-Positive Breast Cancer Treated with Neoadjuvant Anti-HER2-Based Therapy. Clin Cancer Res, 27:3116-3125, 2021
10. Watase C, Shiino S, Shimoi T, Noguchi E, Kaneda T, Yamamoto Y, Yonemori K, Takayama S, Suto A. Breast Cancer Brain Metastasis-Overview of Disease State, Treatment Options and Future Perspectives. Cancers (Basel), 13:2021
11. Yoshida H, Nishikawa T, Matsumoto K, Mori M, Hirashima Y, Takehara K, Ariyoshi K, Hasegawa K, Yonemori K. Histopathological features of HER2 overexpression in uterine carcinosarcoma: proposal for requirements in HER2 testing for targeted therapy. Virchows Arch, 478:1161-1171, 2021
12. Takeyasu Y, Okuma HS, Kojima Y, Nishikawa T, Tanioka M, Sudo K, Shimoi T, Noguchi E, Arakawa A, Mori T, Sunami K, Kubo T, Kohno T, Akihiko Y, Yamamoto N, Yonemori K. Impact of ALK Inhibitors in Patients With ALK-Rearranged Nonlung Solid Tumors. JCO Precis Oncol, 5:2021
13. Turnbull AK, Selli C, Martinez-Perez C, Fernando A, Renshaw L, Keys J, Figueroa JD, He X, Tanioka M, Munro AF, Murphy L, Fawkes A, Clark R, Coutts A, Perou CM, Carey LA, Dixon JM, Sims AH. Unlocking the transcriptomic potential of formalin-fixed paraffin embedded clinical tissues: comparison of gene expression profiling approaches. BMC Bioinformatics, 21:30, 2020
14. Ebata T, Yonemori K, Nishikawa T, Sudo K, Shimomura A, Noguchi E, Fujiwara Y, Kato T, Hasegawa K, Fujiwara K, Tamura K. Treatment Outcome of Second-Line Chemotherapy for Gynecologic Carcinosarcoma. Oncology, 98:699-705, 2020
15. Kato MK, Yunokawa M, Bun S, Shimoi T, Yonemori K, Miyasaka N, Kato T, Tamura K. Treatment strategies for recurrent ovarian cancer in older adult patients in Japan: a study based on real-world data. J Cancer Res Clin Oncol, 146:1335-1341, 2020
16. Kawachi A, Yamashita S, Okochi-Takada E, Hirakawa A, Tsuda H, Shimomura A, Kojima Y, Yonemori K, Fujiwara Y, Kinoshita T, Ushijima T, Tamura K. BRCA1 promoter methylation in breast cancer patients is associated with response to olaparib/eribulin combination therapy. Breast Cancer Res Treat, 181:323-329, 2020
17. Mizuno T, Kojima Y, Yonemori K, Yoshida H, Sugiura Y, Ohtake Y, Okuma HS, Nishikawa T, Tanioka M, Sudo K, Shimomura A, Noguchi E, Kato T, Shimoi T, Uno M, Ishikawa M, Fujiwara Y, Ohe Y, Tamura K. HER3 protein expression as a risk factor for post-operative recurrence in patients with early-stage adenocarcinoma and adenosquamous carcinoma of the cervix. Oncol Lett, 20:38, 2020
18. Mizuno T, Kojima Y, Yonemori K, Yoshida H, Sugiura Y, Ohtake Y, Okuma HS, Nishikawa T, Tanioka M, Sudo K, Shimomura A, Noguchi E, Kato T, Shimoi T, Uno M, Ishikawa M, Fujiwara Y, Ohe Y, Tamura K. Neoadjuvant chemotherapy promotes the expression of HER3 in patients with ovarian cancer. Oncol Lett, 20:336, 2020
19. Ryu S, Ohuchi M, Yagishita S, Shimoi T, Yonemori K, Tamura K, Fujiwara Y, Hamada A. Visualization of the distribution of nanoparticle-formulated AZD2811 in mouse tumor model using matrix-assisted laser desorption ionization mass spectrometry imaging. Sci Rep, 10:15535, 2020
20. Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T. The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast Cancer, 27:322-331, 2020
21. Shimoi T, Sagara Y, Hara F, Toyama T, Iwata H. First-line endocrine therapy for postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer: a systematic review and meta-analysis. Breast Cancer, 27:340-346, 2020
22. Takamizawa S, Ishiki H, Shimoi T, Shimizu M, Satomi E. Neoadjuvant Cisplatin in BRCA Carriers With HER2-Negative Breast Cancer. J Clin Oncol, 38:2699-2700, 2020
23. Watanabe T, Honda T, Totsuka H, Yoshida M, Tanioka M, Shiraishi K, Shimada Y, Arai E, Ushiama M, Tamura K, Yoshida T, Kanai Y, Kohno T. Simple prediction model for homologous recombination deficiency in breast cancers in adolescents and young adults. Breast Cancer Res Treat, 182:491-502, 2020
24. Watanuki R, Shimomura A, Yazaki S, Noda-Narita S, Sumiyoshi-Okuma H, Nishikawa T, Tanioka M, Sudo K, Shimoi T, Noguchi E, Yonemori K, Tamura K. Survival outcomes in patients with human epidermal growth factor receptor 2 positive metastatic breast cancer administered a therapy following trastuzumab emtansine treatment. Medicine (Baltimore), 99:e22331, 2020
