Annual Report 2020
Department of Thoracic Oncology
Yuichiro Ohe, Noboru Yamamoto, Hidehito Horinouchi, Yasushi Goto, Tatsuya Yoshida, Yusuke Okuma, Yuki Shinno, Ken Masuda, Yuji Matsumoto, Masayuki Shirasawa, Sayaka Arakawa, Keisuke Baba, Ryoko Higashiyama
Introduction
Lung cancer is the leading cause of cancer death in Japan and worldwide. The incidence of lung cancer in Japan is still increasing, especially among the elderly. The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The goals of the department are to provide the highest quality treatment and to establish new effective treatments against lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance toward our patients through multidisciplinary care, the staff members of the department work closely with thoracic surgeons, radiation oncologists, pathologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. The department includes nine staff physicians. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
Table 1. Number of patients in 2020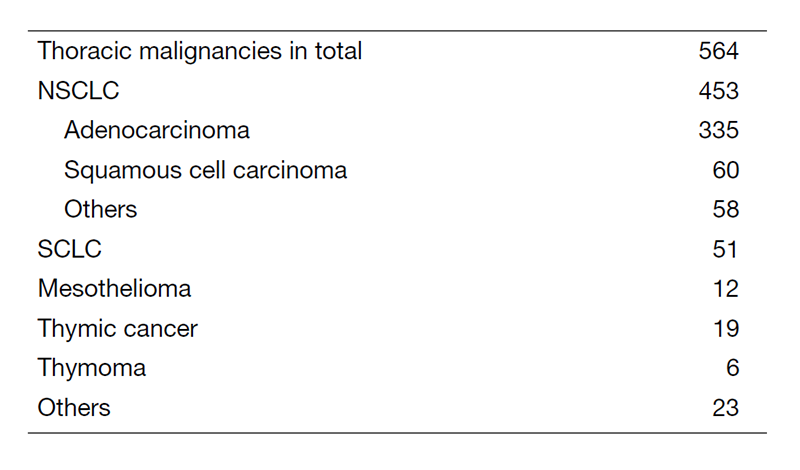

The Team and What We Do
The staff physicians attend to outpatient services for thoracic diseases, and the department has approximately 60 beds in the hospital. Inpatient care is performed by six teams. Each team consists of one staff physician and one or two residents and/or trainee doctors. Case conferences are scheduled every Monday and Tuesday morning. Protocol conference and the journal club are scheduled every Monday afternoon and Thursday morning, respectively.
A total of 564 new patients started treatment in 2020, and the backgrounds and initial treatments of these patients are shown in Tables 1 and 2. The initial treatments included chemotherapy in 294 cases, adjuvant chemotherapy after surgery in 53, chemoradiotherapy in 93, curative radiotherapy in 9, and supportive care including palliative radiotherapy in 46. Survival of lung cancer patients treated in 2011-2015 in our department is shown in Table 3.
Table 2. Type of procedure in 2020

Table 3. Survival of lung cancer patients treated in 2011-2015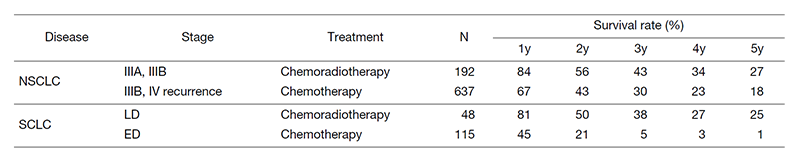

Research activities
Research activities of the department can be classified into four categories: (1) multi-institutional phase III studies to establish new standard treatments against lung cancer; (2) phase I and phase II studies to evaluate new anticancer drugs; (3) pharmacokinetic and pharmacodynamic (PK/PD) studies to investigate interpatient variability, optimal administration schedules and drug-drug interactions; and (4) translational research using clinical samples from bench to bedside or from bedside to bench for the development of innovative treatment strategies.
Clinical trials
The department is currently conducting and participating in multi-institutional phase III studies to establish new standard treatments against lung cancer including the Japan Clinical Oncology Group (JCOG) trials and global trials conducted by pharmaceutical companies. Four JCOG phase III studies are ongoing, namely, JCOG1404 (AGAIN), a phase III study for EGFR mutation-positive NSCLC, JCOG1701, a phase III study of immune checkpoint inhibitors to evaluate optimal treatment period, JCOG1408, a phase III study of SBRT for c-stage IA NSCLC and JCOG1914, a phase III of chemoradiotherapy for elderly locally advanced NSCLC. Patients accrual of JCOG 1404 have been completed in October 2020. The department is also participating in a nationwide screening project of lung cancer with rare driver mutation (LC-SCRUM). The department has conducted many clinical trials using TKIs and immune checkpoint inhibitors.
Education
In 2020, 4 chief residents, 12 residents, 4 trainee doctors, and 1 trainee doctor from overseas have joined the department. A monthly research conference is held to discuss clinical and translational research conducted by young doctors.
Future Prospects
Recent progression of lung cancer treatment is very rapid. Driver gene alteration targeted therapy including EGFR-TKIs for EGFR mutation positive lung cancer, ALK inhibitors for ALK fusion gene-positive lung cancer, ROS1 inhibitors for ROS1 fusion gene-positive lung cancer, BRAF plus MEK inhibitor for BRAF V600E positive lung cancer, NTRK inhibitors for NTRK fusion-positive lung cancer, RET inhibitor for RET fusion-positive lung cancer, and MET inhibitors for MET exon 14 skipping mutation-positive lung cancer are already established as a standard treatment. Other rare driver gene alterations including HER2, KRAS(G12C), and NRG will be good targets for lung cancer treatment. Immune checkpoint inhibitors, anti-PD-1 Ab and anti-PD-L1 Ab plus chemotherapy have been established as a standard first-line treatment for NSCLC. A combination of anti-PD-1 Ab and anti-CTLA-4 Ab with or without chemotherapy have also been established as a new standard treatment for advanced NSCLC. Anti-PD-L1 Ab, durvalumab will also be established as a standard treatment for stage III NSCLC after chemoradiotherapy. An immune checkpoint inhibitor will also be an incorporated treatment for early-stage lung cancer with surgery or SBRT.
List of papers published in 2020
Journal
1. Shirasawa M, Yoshida T, Horinouchi H, Kitano S, Arakawa S, Matsumoto Y, Shinno Y, Okuma Y, Goto Y, Kanda S, Watanabe R, Yamamoto N, Watanabe SI, Ohe Y, Motoi N. Prognostic impact of peripheral blood neutrophil to lymphocyte ratio in advanced-stage pulmonary large cell neuroendocrine carcinoma and its association with the immune-related tumour microenvironment. Br J Cancer, 124:925-932, 2021
2. Kenmotsu H, Niho S, Tsuboi M, Wakabayashi M, Eba J, Asamura H, Ohe Y, Watanabe SI. Reply to J. L. Derks et al. J Clin Oncol, 39:1509-1510, 2021
3. Shirasawa M, Yoshida T, Takayanagi D, Shiraishi K, Yagishita S, Sekine K, Kanda S, Matsumoto Y, Masuda K, Shinno Y, Okuma Y, Goto Y, Horinouchi H, Hamada A, Kohno T, Yamamoto N, Watanabe SI, Ohe Y, Motoi N. Activity and Immune Correlates of Programmed Death-1 Blockade Therapy in Patients With Advanced Large Cell Neuroendocrine Carcinoma. Clin Lung Cancer, 2021
4. Nishida T, Matsumoto Y, Sasada S, Tanaka M, Nakai T, Fukai R, Ohe Y, Watanabe SI, Motoi N. Feasibility study of cryobiopsy for practical pathological diagnosis of primary lung cancer including immunohistochemical assessment. Jpn J Clin Oncol, 51:271-278, 2021
5. Takeyasu Y, Yoshida T, Shibaki R, Matsumoto Y, Goto Y, Kanda S, Horinouchi H, Yamamoto N, Motoi N, Ohe Y. Differential Efficacy of Pembrolizumab According to Metastatic Sites in Patients With PD-L1 Strongly Positive (TPS ≧ 50%) NSCLC. Clin Lung Cancer, 22:127-133.e3, 2021
6. Nishio M, Yoshida T, Kumagai T, Hida T, Toyozawa R, Shimokawaji T, Goto K, Nakagawa K, Ohe Y, Seto T, Kudou K, Asato T, Zhang P, Yamamoto N. Brigatinib in Japanese Patients With ALK-Positive NSCLC Previously Treated With Alectinib and Other Tyrosine Kinase Inhibitors: Outcomes of the Phase 2 J-ALTA Trial. J Thorac Oncol, 16:452-463, 2021
7. Shimoyama R, Omori S, Nomura S, Kenmotsu H, Takahashi T, Harada H, Ishikura S, Mizutani T, Ando M, Kataoka T, Fukuda H, Ohe Y. A multi-institutional randomized phase III study comparing weekly carboplatin plus nab-paclitaxel and daily low-dose carboplatin as regimens for concurrent chemoradiotherapy in elderly patients with unresectable locally advanced non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG1914. Jpn J Clin Oncol, 51:836-841, 2021
8. Baba K, Yoshida T, Shiotsuka M, Kobayashi O, Iwata S, Ohe Y. Rapid development of pulmonary Mycobacterium avium infection during chemoradiotherapy followed by durvalumab treatment in a locally advanced NSCLC patient. Lung Cancer, 153:182-183, 2021
9. Watanabe S, Goto Y, Yasuda H, Kohno T, Motoi N, Ohe Y, Nishikawa H, Kobayashi SS, Kuwano K, Togashi Y. HSP90 inhibition overcomes EGFR amplification-induced resistance to third-generation EGFR-TKIs. Thorac Cancer, 12:631-642, 2021
10. Tanimoto A, Matsumoto S, Takeuchi S, Arai S, Fukuda K, Nishiyama A, Yoh K, Ikeda T, Furuya N, Nishino K, Ohe Y, Goto K, Yano S. Proteasome Inhibition Overcomes ALK-TKI Resistance in ALK-Rearranged/TP53-Mutant NSCLC via Noxa Expression. Clin Cancer Res, 27:1410-1420, 2021
11. Shaikh FY, White JR, Gills JJ, Hakozaki T, Richard C, Routy B, Okuma Y, Usyk M, Pandey A, Weber JS, Ahn J, Lipson EJ, Naidoo J, Pardoll DM, Sears CL. A Uniform Computational Approach Improved on Existing Pipelines to Reveal Microbiome Biomarkers of Nonresponse to Immune Checkpoint Inhibitors. Clin Cancer Res, 27:2571-2583, 2021
12. Egami S, Kawazoe H, Hashimoto H, Uozumi R, Arami T, Sakiyama N, Ohe Y, Nakada H, Aomori T, Ikemura S, Fukunaga K, Yamaguchi M, Nakamura T. Peripheral blood biomarkers predict immune-related adverse events in non-small cell lung cancer patients treated with pembrolizumab: a multicenter retrospective study. J Cancer, 12:2105-2112, 2021
13. Oya Y, Yoshida T, Asada K, Oguri T, Inui N, Morikawa S, Ito K, Kimura T, Kunii E, Matsui T, Kubo A, Kato T, Abe T, Tsuda T, Hida T. Clinical utility of liquid biopsy for EGFR driver, T790M mutation and EGFR amplification in plasma in patients with acquired resistance to afatinib. BMC Cancer, 21:57, 2021
14. Saka H, Nishio M, Hida T, Nakagawa K, Sakai H, Nogami N, Atagi S, Takahashi T, Horinouchi H, Takenoyama M, Katakami N, Tanaka H, Takeda K, Satouchi M, Isobe H, Maemondo M, Goto K, Hirashima T, Minato K, Yada N, Tamura T. Five-year follow-up results from phase II studies of nivolumab in Japanese patients with previously treated advanced non-small cell lung cancer: pooled analysis of the ONO-4538-05 and ONO-4538-06 studies. Jpn J Clin Oncol, 51:106-113, 2021
15. Masuda K, Horinouchi H, Tanaka M, Higashiyama R, Shinno Y, Sato J, Matsumoto Y, Okuma Y, Yoshida T, Goto Y, Yamamoto N, Ohe Y. Efficacy of anti-PD-1 antibodies in NSCLC patients with an EGFR mutation and high PD-L1 expression. J Cancer Res Clin Oncol, 147:245-251, 2021
16. Hibino H, Makino Y, Sakiyama N, Makihara-Ando R, Hashimoto H, Akiyoshi T, Imaoka A, Fujiwara Y, Ohe Y, Yamaguchi M, Ohtani H. Exacerbation of atrioventricular block associated with concomitant use of amlodipine and aprepitant in a lung cancer patient: A case report. Int J Clin Pharmacol Ther, 59:328-332, 2021
17. Arakawa S, Yoshida T, Shirasawa M, Takayanagi D, Yagishita S, Motoi N, Ohe Y. RB1 loss induced small cell lung cancer transformation as acquired resistance to pembrolizumab in an advanced NSCLC patient. Lung Cancer, 151:101-103, 2021
18. Takahashi T, Umeguchi H, Tateishi A, Yoshida T, Motoi N, Ohe Y. Disease flare of leptomeningeal metastases without radiological and cytological findings after the discontinuation of osimertinib. Lung Cancer, 151:1-4, 2021
19. Kashihara T, Nakayama Y, Ito K, Kubo Y, Okuma K, Shima S, Nakamura S, Takahashi K, Inaba K, Murakami N, Igaki H, Ohe Y, Kusumoto M, Itami J. Usefulness of Simple Original Interstitial Lung Abnormality Scores for Predicting Radiation Pneumonitis Requiring Steroidal Treatment After Definitive Radiation Therapy for Patients With Locally Advanced Non-Small Cell Lung Cancer. Adv Radiat Oncol, 6:100606, 2021
20. Mizuno T, Kojima Y, Yonemori K, Yoshida H, Sugiura Y, Ohtake Y, Okuma HS, Nishikawa T, Tanioka M, Sudo K, Shimomura A, Noguchi E, Kato T, Shimoi T, Uno M, Ishikawa M, Fujiwara Y, Ohe Y, Tamura K. HER3 protein expression as a risk factor for post-operative recurrence in patients with early-stage adenocarcinoma and adenosquamous carcinoma of the cervix. Oncol Lett, 20:38, 2020
21. Mizuno T, Kojima Y, Yonemori K, Yoshida H, Sugiura Y, Ohtake Y, Okuma HS, Nishikawa T, Tanioka M, Sudo K, Shimomura A, Noguchi E, Kato T, Shimoi T, Uno M, Ishikawa M, Fujiwara Y, Ohe Y, Tamura K. Neoadjuvant chemotherapy promotes the expression of HER3 in patients with ovarian cancer. Oncol Lett, 20:336, 2020
22. Shirasawa M, Yoshida T, Matsumoto Y, Shinno Y, Okuma Y, Goto Y, Horinouchi H, Yamamoto N, Watanabe SI, Ohe Y, Motoi N. Impact of chemoradiotherapy on the immune-related tumour microenvironment and efficacy of anti-PD-(L)1 therapy for recurrences after chemoradiotherapy in patients with unresectable locally advanced non-small cell lung cancer. Eur J Cancer, 140:28-36, 2020
23. Kenmotsu H, Niho S, Tsuboi M, Wakabayashi M, Ishii G, Nakagawa K, Daga H, Tanaka H, Saito H, Aokage K, Takahashi T, Menju T, Kasai T, Yoshino I, Minato K, Okada M, Eba J, Asamura H, Ohe Y, Watanabe SI. Randomized Phase III Study of Irinotecan Plus Cisplatin Versus Etoposide Plus Cisplatin for Completely Resected High-Grade Neuroendocrine Carcinoma of the Lung: JCOG1205/1206. J Clin Oncol, 38:4292-4301, 2020
24. Kobayashi AK, Horinouchi H, Nakayama Y, Ohe Y, Yotsukura M, Uchida S, Asakura K, Yoshida Y, Nakagawa K, Watanabe SI. Salvage surgery after chemotherapy and/or radiotherapy including SBRT and proton therapy: A consecutive analysis of 38 patients. Lung Cancer, 145:105-110, 2020
25. Hosoba R, Makita S, Shiotsuka M, Kobayashi O, Nakano K, Muroya M, Okada N, Suzuki M, Ida H, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Maeshima AM, Matsushita H, Yamamoto N, Ohe Y, Iwata S, Izutsu K. COVID-19 pneumonia in a patient with adult T-cell leukemia-lymphoma. J Clin Exp Hematop, 60:174-178, 2020
26. Gemma A, Kusumoto M, Sakai F, Endo M, Kato T, Saito Y, Baba T, Sata M, Yamaguchi O, Yabuki Y, Nogi Y, Jinushi M, Sakamoto K, Sugeno M, Tamura R, Tokimoto T, Ohe Y. Real-World Evaluation of Factors for Interstitial Lung Disease Incidence and Radiologic Characteristics in Patients With EGFR T790M-positive NSCLC Treated With Osimertinib in Japan. J Thorac Oncol, 15:1893-1906, 2020
27. Inaba-Higashiyama R, Yoshida T, Jo H, Shirasawa M, Motoi N, Ohe Y. Clinical outcomes of pembrolizumab therapy in advanced-NSCLC patients with poor performance status (≧3) and high PD-L1 expression (TPS ≧50%): A case series. Thorac Cancer, 11:3618-3621, 2020
28. Murakami S, Shibaki R, Matsumoto Y, Yoshida T, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Ohe Y. Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody. Thorac Cancer, 11:3585-3595, 2020
29. Kawada M, Nobeyama Y, Goto Y, Nakama K, Yamazaki N, Asahina A. Absence of toxic epidermal necrolysis recurrence with pembrolizumab re-challenge in a patient with a positive lymphocyte transformation test. J Dermatol, 47:e424-e425, 2020
30. Satouchi M, Nosaki K, Takahashi T, Nakagawa K, Aoe K, Kurata T, Sekine A, Horiike A, Fukuhara T, Sugawara S, Umemura S, Saka H, Okamoto I, Yamamoto N, Sakai H, Kishi K, Katakami N, Horinouchi H, Hida T, Okamoto H, Atagi S, Ohira T, Han SR, Noguchi K, Ebiana V, Hotta K. First-line pembrolizumab vs chemotherapy in metastatic non-small-cell lung cancer: KEYNOTE-024 Japan subset. Cancer Sci, 111:4480-4489, 2020
31. Miura S, Yamanaka T, Kato T, Ikeda S, Horinouchi H, Ichihara E, Kanazu M, Takiguchi Y, Tanaka K, Goto Y, Sata M, Hagiwara K, Okamoto H, Tanaka H. Treatment Rationale and Design of a Phase III Study of Afatinib or Chemotherapy in Patients with Non-small-cell Lung Cancer Harboring Sensitizing Uncommon Epidermal Growth Factor Receptor Mutations (ACHILLES/TORG1834). Clin Lung Cancer, 21:e592-e596, 2020
32. Hakozaki T, Hosomi Y, Kitadai R, Kitagawa S, Okuma Y. Efficacy of immune checkpoint inhibitor monotherapy for patients with massive non-small-cell lung cancer. J Cancer Res Clin Oncol, 146:2957-2966, 2020
33. Fujisawa D, Umemura S, Okizaki A, Satomi E, Yamaguchi T, Miyaji T, Mashiko T, Kobayashi N, Kinoshita H, Mori M, Morita T, Uchitomi Y, Goto K, Ohe Y, Matsumoto Y. Nurse-led, screening-triggered, early specialised palliative care intervention programme for patients with advanced lung cancer: study protocol for a multicentre randomised controlled trial. BMJ Open, 10:e037759, 2020
34. Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, Mazieres J, Kim DW, Mok T, Polli A, Thurm H, Calella AM, Peltz G, Solomon BJ. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med, 383:2018-2029, 2020
35. Hakozaki T, Hosomi Y, Shimizu A, Kitadai R, Mirokuji K, Okuma Y. Polypharmacy as a prognostic factor in older patients with advanced non-small-cell lung cancer treated with anti-PD-1/PD-L1 antibody-based immunotherapy. J Cancer Res Clin Oncol, 146:2659-2668, 2020
36. Okuma Y, Ko R, Shukuya T, Tateishi K, Imai H, Iwasawa S, Miyauchi E, Kojima T, Fujita Y, Hino T, Yamanda S, Suzuki T, Fukuizumi A, Sakakibara T, Harada T, Morita S, Kobayashi K, Nukiwa T, Takahashi K. Prognostic factors for patients with metastatic or recurrent thymic carcinoma receiving palliative-intent chemotherapy. Lung Cancer, 148:122-128, 2020
37. Hakozaki T, Richard C, Elkrief A, Hosomi Y, Benla?faoui M, Mimpen I, Terrisse S, Derosa L, Zitvogel L, Routy B, Okuma Y. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Immunol Res, 8:1243-1250, 2020
38. Ito K, Murotani K, Kubo A, Kunii E, Taniguchi H, Shindoh J, Asada K, Imaizumi K, Takahashi K, Karayama M, Okuno M, Inui N, Hataji O, Morikawa S, Hayai S, Suda T, Abe T, Tsuda T, Yamagichi T, Kimura T, Oya Y, Yoshida T, Hida T. Propensity score analysis of overall survival between first- and second-generation EGFR-TKIs using real-world data. Cancer Sci, 111:3705-3713, 2020
39. Okuma Y, Goto Y, Ohyanagi F, Sunami K, Nakahara Y, Kitazono S, Kudo K, Tambo Y, Kanda S, Yanagitani N, Horiike A, Horinouchi H, Fujiwara Y, Nokihara H, Yamamoto N, Nishio M, Ohe Y, Hosomi Y. Phase II trial of S-1 treatment as palliative-intent chemotherapy for previously treated advanced thymic carcinoma. Cancer Med, 9:7418-7427, 2020
40. Goto Y. Current Understanding and Biomarker Application of Programmed Death-Ligand 1 Expression in Tumors. J Thorac Oncol, 15:1392-1393, 2020
41. Onishi Y, Kusumoto M, Goto Y, Kaku S, Motoi N. Epithelioid hemangioendothelioma of the lung: CT findings and clinical course of 35 cases. Jpn J Clin Oncol, 50:1195-1200, 2020
42. Horinouchi H, Atagi S, Oizumi S, Ohashi K, Kato T, Kozuki T, Seike M, Sone T, Sobue T, Tokito T, Harada H, Maeda T, Mio T, Shirosaka I, Hattori K, Shin E, Murakami H. Real-world outcomes of chemoradiotherapy for unresectable Stage III non-small cell lung cancer: The SOLUTION study. Cancer Med, 9:6597-6608, 2020
43. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, McCoach CE, Gautschi O, Besse B, Cho BC, Peled N, Weiss J, Kim YJ, Ohe Y, Nishio M, Park K, Patel J, Seto T, Sakamoto T, Rosen E, Shah MH, Barlesi F, Cassier PA, Bazhenova L, De Braud F, Garralda E, Velcheti V, Satouchi M, Ohashi K, Pennell NA, Reckamp KL, Dy GK, Wolf J, Solomon B, Falchook G, Ebata K, Nguyen M, Nair B, Zhu EY, Yang L, Huang X, Olek E, Rothenberg SM, Goto K, Subbiah V. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med, 383:813-824, 2020
44. Arakawa S, Yoshida T, Nakayama Y, Motoi N, Ohe Y. Small Cell Cancer Transformation of Lung Adenocarcinoma During Durvalumab Treatment After Chemoradiotherapy. J Thorac Oncol, 15:e145-e146, 2020
45. Zenke Y, Niho S, Umemura S, Ishihara M, Seki N, Nogami N, Hosomi Y, Shimokawa T, Tokito T, Goto Y, Miura Y, Saito H, Hida N, Ikeda S, Tanaka H, Furuya N, Misumi T, Yamanaka T, Ohe Y, Okamoto H. Phase I/II study of carboplatin plus weekly nab-paclitaxel in patients aged ≧75 years with squamous-cell lung cancer: TORG1322. Lung Cancer, 146:182-188, 2020
46. Ohe Y, Kato T, Sakai F, Kusumoto M, Endo M, Saito Y, Baba T, Sata M, Yamaguchi O, Sakamoto K, Sugeno M, Tamura R, Tokimoto T, Shimizu W, Gemma A. Real-world use of osimertinib for epidermal growth factor receptor T790M-positive non-small cell lung cancer in Japan. Jpn J Clin Oncol, 50:909-919, 2020
47. Seto T, Hayashi H, Satouchi M, Goto Y, Niho S, Nogami N, Hida T, Takahashi T, Sakakibara-Konishi J, Morise M, Nagasawa T, Suzuki M, Ohkura M, Fukuhara K, Thurm H, Peltz G, Nishio M. Lorlatinib in previously treated anaplastic lymphoma kinase-rearranged non-small cell lung cancer: Japanese subgroup analysis of a global study. Cancer Sci, 111:3726-3738, 2020
48. Jinnai S, Yamazaki N, Hirano Y, Sugawara Y, Ohe Y, Hamamoto R. The Development of a Skin Cancer Classification System for Pigmented Skin Lesions Using Deep Learning. Biomolecules, 10:2020
49. Okishio K, Morita R, Shimizu J, Saito H, Sakai H, Kim YH, Hataji O, Yomota M, Nishio M, Aoe K, Kanai O, Kumagai T, Kibata K, Tsukamoto H, Oizumi S, Fujimoto D, Tanaka H, Mizuno K, Masuda T, Kozuki T, Haku T, Suzuki H, Okamoto I, Hoshiyama H, Yada N, Ohe Y. Nivolumab treatment of elderly Japanese patients with non-small cell lung cancer: subanalysis of a real-world retrospective observational study (CA209-9CR). ESMO Open, 5:2020
50. Ichikawa J, Yoshida T, Isser A, Laino AS, Vassallo M, Woods D, Kim S, Oelke M, Jones K, Schneck JP, Weber JS. Rapid Expansion of Highly Functional Antigen-Specific T Cells from Patients with Melanoma by Nanoscale Artificial Antigen-Presenting Cells. Clin Cancer Res, 26:3384-3396, 2020
51. Kashima J, Okuma Y. Bridging over troubled waters: the doubling time and histological subtypes of thymic epithelial tumors. J Thorac Dis, 12:3886-3889, 2020
52. Nomura S, Goto Y, Mizutani T, Kataoka T, Kawai S, Okuma Y, Murakami H, Tanaka K, Ohe Y. A randomized phase III study comparing continuation and discontinuation of PD-1 pathway inhibitors for patients with advanced non-small-cell lung cancer (JCOG1701, SAVE study). Jpn J Clin Oncol, 50:821-825, 2020
53. Goto Y, Yamamoto N, Masters ET, Kikkawa H, Mardekian J, Wiltshire R, Togo K, Ohe Y. Treatment Sequencing in Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer in Japan: A Real-World Observational Study. Adv Ther, 37:3311-3323, 2020
54. Shibaki R, Murakami S, Shinno Y, Matsumoto Y, Yoshida T, Goto Y, Kanda S, Horinouchi H, Fujiwara Y, Yamamoto N, Yamamoto N, Ohe Y. Predictive value of serum VEGF levels for elderly patients or for patients with poor performance status receiving anti-PD-1 antibody therapy for advanced non-small-cell lung cancer. Cancer Immunol Immunother, 69:1229-1236, 2020
55. Sato J, Satouchi M, Itoh S, Okuma Y, Niho S, Mizugaki H, Murakami H, Fujisaka Y, Kozuki T, Nakamura K, Nagasaka Y, Kawasaki M, Yamada T, Machida R, Kuchiba A, Ohe Y, Yamamoto N. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol, 21:843-850, 2020
56. Schwartzberg LS, Horinouchi H, Chan D, Chernilo S, Tsai ML, Isla D, Escriu C, Bennett JP, Clark-Langone K, Svedman C, Tomasini P. Liquid biopsy mutation panel for non-small cell lung cancer: analytical validation and clinical concordance. NPJ Precis Oncol, 4:15, 2020
57. Kanda S, Ohe Y, Goto Y, Horinouchi H, Fujiwara Y, Nokihara H, Yamamoto N, Yamamoto T, Tamura T. Five-year safety and efficacy data from a phase Ib study of nivolumab and chemotherapy in advanced non-small-cell lung cancer. Cancer Sci, 111:1933-1942, 2020
58. Kawai S, Suzuki H, Okuma Y. Durvalumab Consolidation Treatment after Chemoradiotherapy for an HIV-Positive Patient with Locally Advanced Non-Small Cell Lung Cancer. Case Rep Oncol, 13:747-753, 2020
59. Sato J, Kitano S, Motoi N, Ino Y, Yamamoto N, Watanabe S, Ohe Y, Hiraoka N. CD20+ tumor-infiltrating immune cells and CD204+ M2 macrophages are associated with prognosis in thymic carcinoma. Cancer Sci, 111:1921-1932, 2020
60. Katsuya Y, Miyake K, Higuchi T, Oshiro H, Sugisawa N, Singh SR, Goto Y, Zhao M, Hoffman RM. Comparison of the Efficacy of EGFR Tyrosine Kinase Inhibitors Erlotinib and Low-dose Osimertinib on a PC-9-GFP EGFR Mutant Non-small-cell Lung Cancer Growing in the Brain of Nude Mice. In Vivo, 34:1027-1030, 2020
61. Ito M, Kanda S, Yoshida T, Okuma Y, Jo H, Fukuhara S, Maeshima AM, Ohe Y. Eltrombopag olamine for refractory immune-related thrombocytopenia induced by pembrolizumab in a non-small cell lung cancer patient. Lung Cancer, 146:362-365, 2020
62. Horinouchi H, Ohe Y. History of Japan Clinical Oncology Group (JCOG) Lung Cancer Study Group. Jpn J Clin Oncol, 50:502-511, 2020
63. Okuno T, Arakawa S, Yoshida T, Ohe Y. Efficacy of osimertinib in a patient with leptomeningeal metastasis and EGFR uncommon S768I mutation. Lung Cancer, 143:95-96, 2020
64. Okamoto I, Nokihara H, Nomura S, Niho S, Sugawara S, Horinouchi H, Azuma K, Yoneshima Y, Murakami H, Hosomi Y, Atagi S, Ozaki T, Horiike A, Fujita Y, Okamoto H, Ando M, Yamamoto N, Ohe Y, Nakagawa K. Comparison of Carboplatin Plus Pemetrexed Followed by Maintenance Pemetrexed With Docetaxel Monotherapy in Elderly Patients With Advanced Nonsquamous Non-Small Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol, 6:e196828, 2020
65. Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, Bunn P, Cascone T, Chaft J, Chen G, Chou TY, Cooper W, Erasmus JJ, Ferreira CG, Goo JM, Heymach J, Hirsch FR, Horinouchi H, Kerr K, Kris M, Jain D, Kim YT, Lopez-Rios F, Lu S, Mitsudomi T, Moreira A, Motoi N, Nicholson AG, Oliveira R, Papotti M, Pastorino U, Paz-Ares L, Pelosi G, Poleri C, Provencio M, Roden AC, Scagliotti G, Swisher SG, Thunnissen E, Tsao MS, Vansteenkiste J, Weder W, Yatabe Y. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol, 15:709-740, 2020
66. Horinouchi H. To combine or not to combine: anti-vascular endothelial growth factor therapies in EGFR mutation positive non-small cell lung cancer. Ann Transl Med, 8:554, 2020
67. Yoshida T, Ichikawa J, Giuroiu I, Laino AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Stephen Hodi F, Weber J. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer, 8:2020
68. Mizuno T, Horinouchi H, Watanabe S, Sato J, Morita R, Murakami S, Goto Y, Kanda S, Fujiwara Y, Yamamoto N, Ohe Y. Number of metastatic organs negatively affects the treatment sequence in patients with EGFR-TKI failure. Thorac Cancer, 11:1038-1044, 2020
69. Tateishi K, Ko R, Shukuya T, Okuma Y, Watanabe S, Kuyama S, Murase K, Tsukita Y, Ashinuma H, Nakagawa T, Uematsu K, Nakao M, Mori Y, Kaira K, Mouri A, Miyabayashi T, Sakashita H, Matsumoto Y, Tanigawa T, Koizumi T, Morita S, Kobayashi K, Nukiwa T, Takahashi K. Clinical Outcomes of Second-Line Chemotherapy in Patients with Previously Treated Advanced Thymic Carcinoma: A Retrospective Analysis of 191 Patients from the NEJ023 Study. Oncologist, 25:e668-e674, 2020
