Annual Report 2020
Department of Urology
Yoshiyuki Matsui, Motokiyo Komiyama, Yasuo Shinoda, Aiko Maejima, Hiroyuki Fujimoto
Introduction
In the Department of Urology, all urogenital malignant diseases, including adrenal cancer, kidney cancer, urothelial cancer, prostate cancer, testicular germ cell tumors, and retroperitoneal sarcoma, are the subject of diagnosis and treatment with comprehensive approaches, including radical surgery, irradiation, and chemotherapy.
The Team and What We Do
The urology team consists of five staff physicians and three residents. In addition, with the participation of a radiation oncologist or interventional radiologist, multidisciplinary treatments for advanced urogenital malignant diseases are performed. Every morning ward round attended by all the staff starts at 7:45 a.m., and a weekly clinical conference is held on Monday evenings. Annual statistics of major treatments are summarized in Table 1.
Major urological malignant diseases are treated according to the following strategies:
1) Renal cell carcinoma: M0, partial or radical nephrectomy (laparoscopic surgery, robotic surgery); M1: systemic treatment with targeted therapy with and/or immune checkpoint inhibitor (ICI) followed by palliative nephrectomy and metastasectomy. A selected small size (less than 3 cm) tumor: cryoablation.
2) Bladder cancer: Ta, T1, Carcinoma in situ (Cis); transurethral resection (TUR), often combined with preoperative or postoperative BCG instillation. T2-T4, radical cystectomy with neoadjuvant chemotherapy by an M-VAC/GC regimen. N+; systemic chemotherapy plus radiation or consolidation surgery; sometimes urinary diversion alone. M+; chemotherapy with an M-VAC /GC or ICI regimen.
3) Prostate cancer: Organ-confined disease, active surveillance, robotic-assisted radical prostatectomy (RARP), irradiation, or endocrine therapy. Specimen-confined disease, extended radical prostatectomy without neoadjuvant endocrine therapy, radiation therapy with endocrine therapy, or endocrine therapy alone. For high risk locally advanced prostate cancer, extended pelvic lymph node dissection was performed. N1; radiation therapy with endocrine therapy. M1; endocrine therapy (vintage or androgen receptor axis target therapy (ARAT)) and palliative radiation if necessary. For castration-refractory disease, ARAT, docetaxel or cabazitaxel chemotherapy, and Ra223 are indicated. PARP inhibitor is applied for castration resistant prostate cancer with BRCA mutation.
4) Testicular germ cell tumor (GCT): Stage I, high inguinal orchiectomy and careful follow-up or adjuvant chemotherapy. Stage II or higher, EP or BEP systemic chemotherapy as the first line. In non-seminoma GCT cases, a salvage operation is performed after induction chemotherapy. In seminoma cases, careful observation rather than surgery is selected if the tumor has shrunk to less than 3 cm.
Table 1. Patients statistics: Major treatment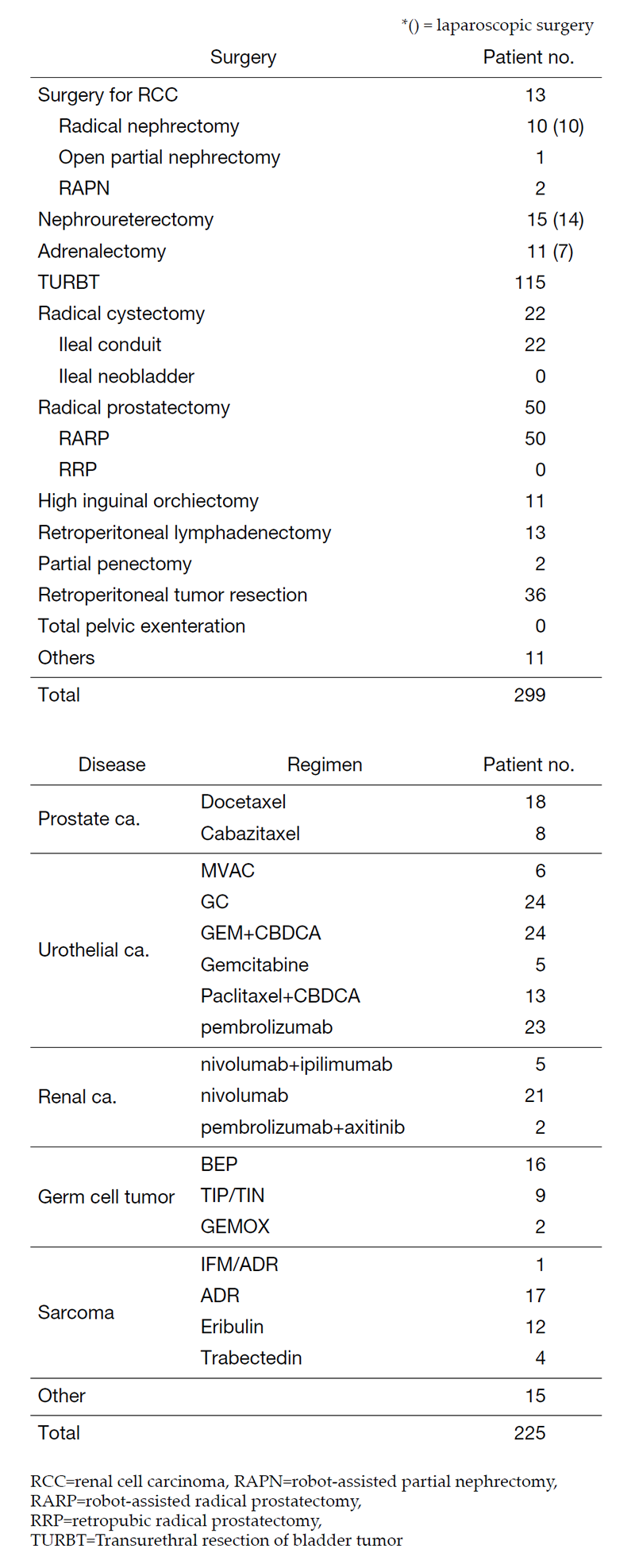

Research activities
We are constantly seeking ways to improve the treatment of malignant urological tumors.
1) Urothelial cancer: A phase III study to confirm the efficacy of pirarubicin in the prevention of bladder recurrence after radical nephroureterectomy for upper tract urothelial carcinoma (JCOG1403) is ongoing. As a second or third-line chemotherapy, a weekly CBDCA + PTX regimen has been adopted.
2) Prostate cancer: A robotic operative method to achieve a complete surgical margin (extended radical prostatectomy) has been established. The significance of lymph node dissection in high risk prostate cancer has been examined in the phase II study: ICG-navigated pelvic LN dissection for robotic assisted laparoscopic prostatectomy.
3) Renal cell carcinoma: A randomized controlled phase III trial on continued or paused PD-1 pathway blockade for patients with advanced renal cell carcinoma (JCOG1905) is ongoing.
4) Testicular GCTs: For advanced and/or refractory cases, a so-called “desperate operation”, which was designed for patients whose tumor markers did not normalize after induction chemotherapy, has been shown to be both efficacious and clinically significant. For CDDP-refractory GCTs, a second line TIP/TIN, and third line IrNd, GEMOX regimen has been adopted.
Clinical trials
We are actively involved in the following mainly ongoing protocol studies:
1) A phase III study: A single early intravesical instillation of pirarubicin in the prevention of bladder recurrence after radical nephroureterectomy for upper tract urothelial carcinoma (JCOG1403)
2) A randomized controlled phase III trial on continued or paused PD-1 pathway blockade for patients with advanced renal cell carcinoma (JCOG1905)
3) A phase III, randomized, open-label, controlled, multicenter, global study of first-line durvalumab in combination with standard of care chemotherapy and durvalumab in combination with tremelimumab and standard of care chemotherapy versus standard of care chemotherapy alone in patients with unresectable locally advanced or metastatic urothelial cancer
4) A phase II, open-label, randomized, multicenter study to evaluate the efficacy and safety of pemigatinib plus pembrolizumab versus pemigatinib alone versus standard of care as first-line treatment for metastatic or unresectable urothelial carcinoma in cisplatin-ineligible participants whose tumors express FGFR3 mutation or rearrangement (FIGHT-205)
5) A phase III randomized trial of belzutifan (MK-6482) versus everolimus in participants with advanced renal cell carcinoma (MK-6482-005)
Education
1) Urological residents learn standard laparoscopic surgery to obtain a certificate for the "Urinary laparoscopic technology certification system".
2) Gynecological or colorectal residents are rotating to our department, for further pelvic surgical training.
Future Prospects
We aim to establish the standard protocol of multimodality treatment for complicated urological malignancy or retroperitoneal sarcoma and to minimize treatment invasiveness by introduction of robotic or laparoscopic surgery.
List of papers published in 2020
Journal
1. Noguchi M, Fujimoto K, Arai G, Uemura H, Hashine K, Matsumoto H, Fukasawa S, Kohjimoto Y, Nakatsu H, Takenaka A, Fujisawa M, Uemura H, Naito S, Egawa S, Fujimoto H, Hinotsu S, Itoh K. A randomized phase III trial of personalized peptide vaccination for castration?resistant prostate cancer progressing after docetaxel. Oncol Rep, 45:159-168, 2021
2. Ohmomo H, Komaki S, Ono K, Sutoh Y, Hachiya T, Arai E, Fujimoto H, Yoshida T, Kanai Y, Sasaki M, Shimizu A. Evaluation of clinical formalin-fixed paraffin-embedded tissue quality for targeted-bisulfite sequencing. Pathol Int, 71:135-140, 2021
3. Kobayashi T, Ito K, Kojima T, Kato M, Kanda S, Hatakeyama S, Matsui Y, Matsushita Y, Naito S, Shiga M, Miyake M, Muro Y, Nakanishi S, Kato Y, Shibuya T, Hayashi T, Yasumoto H, Yoshida T, Uemura M, Taoka R, Kamiyama M, Ogawa O, Kitamura H, Nishiyama H. Risk stratification for the prognosis of patients with chemoresistant urothelial cancer treated with pembrolizumab. Cancer Sci, 112:760-773, 2021
4. Miyake M, Iida K, Nishimura N, Miyamoto T, Fujimoto K, Tomida R, Matsumoto K, Numakura K, Inokuchi J, Morizane S, Yoneyama T, Matsumura Y, Abe T, Inoue M, Yamada T, Terada N, Hirao S, Uemura M, Matsushita Y, Taoka R, Kobayashi T, Kojima T, Matsui Y, Kitamura H, Nishiyama H. Non-maintenance intravesical Bacillus Calmette-Gu?rin induction therapy with eight doses in patients with high- or highest-risk non-muscle invasive bladder cancer: a retrospective non-randomized comparative study. BMC Cancer, 21:266, 2021
5. Ito K, Kobayashi M, Komiyama M, Naito S, Nishimura K, Yonese J, Hashine K, Saito S, Arai G, Shinohara M, Masumori N, Shimizu N, Satoh T, Yamauchi A, Tochigi T, Takezawa Y, Fujimoto H, Yokomizo A, Kakimoto KI, Fukui I, Karasawa K, Tsukamoto T, Nozaki M, Hasumi M, Ishiyama H, Ohtani M, Kuwahara M, Harada M, Ohashi Y, Kotake T, Kakizoe T, Suzuki K, Yamanaka H. Oncological outcomes for patients with locally advanced prostate cancer treated with neoadjuvant endocrine and external-beam radiation therapy followed by adjuvant continuous/intermittent endocrine therapy in an open-label, randomized, phase 3 trial. Cancer, 126:3961-3971, 2020
6. Tsuchida K, Inaba K, Kashihara T, Murakami N, Okuma K, Takahashi K, Igaki H, Nakayama Y, Maejima A, Shinoda Y, Matsui Y, Komiyama M, Fujimoto H, Ito Y, Sumi M, Nakano T, Itami J. Clinical outcomes of definitive whole pelvic radiotherapy for clinical lymph node metastatic prostate cancer. Cancer Med, 9:6629-6637, 2020
7. Matsumoto H, Shiraishi K, Azuma H, Inoue K, Uemura H, Eto M, Ohyama C, Ogawa O, Kikuchi E, Kitamura H, Shinohara N, Takahashi S, Tsuzuki T, Nakagawa M, Narumi Y, Nishiyama H, Habuchi T, Hinotsu S, Fujii Y, Fujimoto K, Fujimoto H, Mizowaki T, Matsuyama H. Clinical Practice Guidelines for Bladder Cancer 2019 update by the Japanese Urological Association: Summary of the revision. Int J Urol, 27:702-709, 2020
8. Matsui Y. Current Multimodality Treatments Against Brain Metastases from Renal Cell Carcinoma. Cancers (Basel), 12:2020
9. Kashihara T, Inaba K, Komiyama M, Nakayama H, Iijima K, Nishioka S, Okamoto H, Kikkawa N, Kubo Y, Shima S, Nakamura S, Takahashi A, Takahashi K, Okuma K, Murakami N, Igaki H, Nakayama Y, Fukunaga A, Matsui Y, Fujimoto H, Itami J. The use of hyperbaric oxygen to treat actinic rectal fistula after SpaceOAR use and radiotherapy for prostate cancer: a case report. BMC Urol, 20:196, 2020
10. Fujimoto M, Arai E, Tsumura K, Yotani T, Yamada Y, Takahashi Y, Maeshima AM, Fujimoto H, Yoshida T, Kanai Y. Establishment of diagnostic criteria for upper urinary tract urothelial carcinoma based on genome-wide DNA methylation analysis. Epigenetics, 15:1289-1301, 2020
