Annual Report 2020
Department of Pediatric Oncology
Chitose Ogawa, Tadashi Kumamoto, Ayumu Arakawa, Masanaka Sugiyama, Yuko Watanabe, Nami Shirakawa, Kayoko Tao, Yuki Nogami
Introduction
Pediatric oncology includes a wide variety of malignancies in children and adolescents such as acute leukemia and malignant lymphoma, as well as solid tumors including osteosarcoma, soft tissue sarcoma, neuroblastoma, liver tumor and retinoblastoma. Many diseases are usually chemo-sensitive and curable with appropriate treatment. The common approach to these diseases is a “risk-adapted therapy” strategy that considers long-term life expectancy. In the Department of Pediatric Oncology, pediatric patients with malignancies are managed by five pediatric oncologists and two pediatric surgeons. Although pediatric oncologists mainly treat and manage patients, we employ a multidisciplinary team approach involving radiation oncologists, orthopedic surgeons, ophthalmologic surgeons and others for the treatment. To achieve treatment completion and optimal quality of hospital life for children, pediatric nurse specialists, teachers, child care staff, psychologists and psychiatrists also join our team. For young patients, educational opportunities ranging from elementary school to high school are available in the pediatric ward, where 10 teachers work daily.
The Team and What We Do
In FY2020, about 90 new patients came to our department and 77 patients have started treatment. The number of patients is shown in Table 1. Our daily activity in the pediatric outpatient clinic includes managing new patients, treating patients with chemotherapy or blood transfusion and providing follow-up care for patients who have completed intensive therapy. Patients undergo multidisciplinary therapy, including surgical removal of the tumor, radiation therapy, chemotherapy, and sometimes stem cell transplantation, as indicated.
A pediatric conference is held every morning, mainly to decide on individual treatment plans. The pediatric staff and trainees discuss various issues regarding pediatric inpatients on daily rounds. Interdepartmental conferences in cooperation with orthopedics, radiation oncology, and palliative care are individually scheduled every 2 weeks.
Table 1. Number of patients between April 2020 and Mach 2021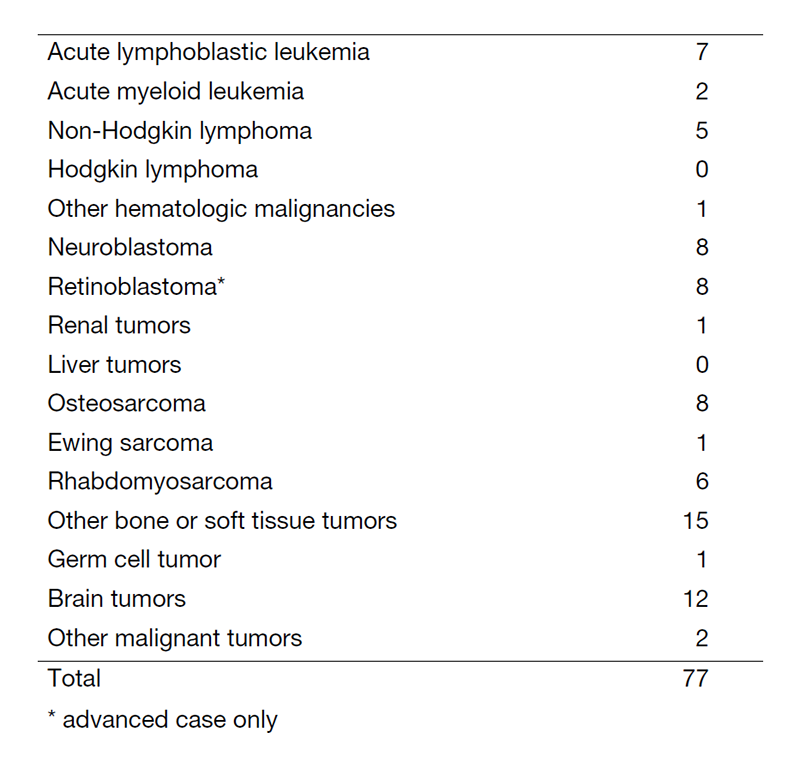

Research activities
1. For newly diagnosed patients, we participate in several multicenter studies conducted by the Japan Children’s Cancer Group (JCCG), including those by the Japan Ewing Sarcoma Study Group (JESS), Rhabdomyosarcoma Study Group (JRSG) and Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG).
2. For relapsed patients, we are actively involved in the development of new drugs and treatments, including off-label and unapproved medications.
3. For individualized treatment, we have initiated the TOP-GEAR project, implementing clinical sequencing for children. A total of 132 samples had been analyzed by the end of March, 2021. Potentially actionable findings were identified in 36 (27%) patients, and 13 (10%) patients have undergone a recommended therapy according to their genetic profiles. Furthermore, through this project, a novel pathway of cancer transmission from mothers with cervical cancer to infants was found and reported in the N Engl J Med.
4. To provide an environment during therapy similar to that before the onset of the patient’s disease, we plan to construct a medical care system through the use of appropriate medical and social resources in the patients' local communities.
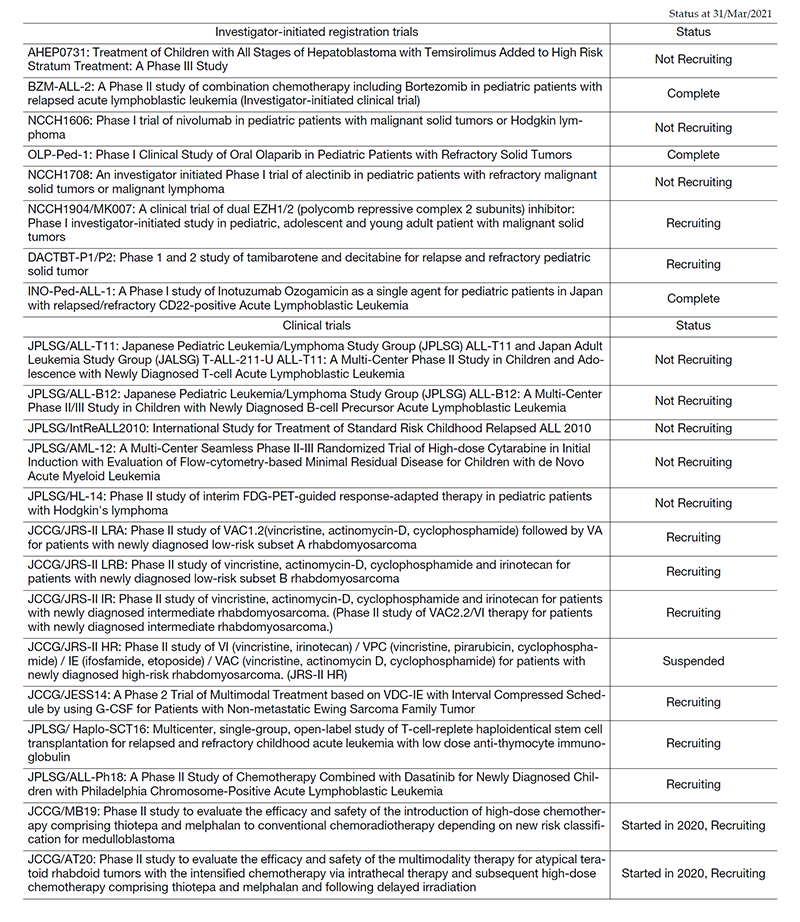
Clinical trials
In 2020, we conducted 22 clinical trials, including early phase trials, an international study and cooperative studies. All clinical trials are listed in Table 2. Among them, eight trials were investigator-initiated, registration-directed clinical trials conducted under the Pharmaceutical Affairs Law in Japan. We conducted two international cooperative trials: IntReALL2010, in collaboration with the International BFM group in Europe, and AHEP 0731, in collaboration with the Children's Oncology Group in the US, which has completed recruitment.
Education
We provide personnel training and education for the diagnosis and management of pediatric hematological malignancies and solid tumors. Residents also learn skills to treat not only newly diagnosed patients but also relapsed or refractory patients through the global standard of therapy. In addition, senior residents acquire abilities to plan studies for new agents and therapies, which we regard as an important role of this center.
Future Prospects
We will promote the development of therapies for pediatric malignancies as a top priority. For this mission, we will plan clinical or registration trials in cooperation with domestic and international centers as a core institution in Japan.
Our other mission is to provide individualized medicine for children with cancer. To this end, we have expanded the number of participants in our center's comprehensive genetic testing project in each pediatric age group. In addition, we will promote clinical trials using molecular targeted agents for pediatric malignancies.
List of papers published in 2020
Journal
1. de Groot AP, Saito Y, Kawakami E, Hashimoto M, Aoki Y, Ono R, Ogahara I, Fujiki S, Kaneko A, Sato K, Kajita H, Watanabe T, Takagi M, Tomizawa D, Koh K, Eguchi M, Ishii E, Ohara O, Shultz LD, Mizutani S, Ishikawa F. Targeting critical kinases and anti-apoptotic molecules overcomes steroid resistance in MLL-rearranged leukaemia. EBioMedicine, 64:103235, 2021
2. Nishimura A, Aoki Y, Ishiwata Y, Ichimura T, Ueyama J, Kawahara Y, Tomoda T, Inoue M, Matsumoto K, Inoue K, Hiroki H, Ono S, Yamashita M, Okano T, Tanaka-Kubota M, Ashiarai M, Miyamoto S, Miyawaki R, Yamagishi C, Tezuka M, Okawa T, Hoshino A, Endo A, Yasuhara M, Kamiya T, Mitsuiki N, Ono T, Isoda T, Yanagimachi M, Tomizawa D, Nagasawa M, Mizutani S, Kajiwara M, Takagi M, Kanegane H, Imai K, Morio T. Hematopoietic Cell Transplantation with Reduced Intensity Conditioning Using Fludarabine/Busulfan or Fludarabine/Melphalan for Primary Immunodeficiency Diseases. J Clin Immunol, 2021
3. Mascarenhas L, Ogawa C, Laetsch TW, Weigel BJ, Bishop MW, Krystal J, Borinstein SC, Slotkin EK, Muscal JA, Hingorani P, Levy DE, Mo G, Shahir A, Wright J, DuBois SG. Phase 1 trial of olaratumab monotherapy and in combination with chemotherapy in pediatric patients with relapsed/refractory solid and central nervous system tumors. Cancer Med, 10:843-856, 2021
4. Umeda K, Miyamura T, Yamada K, Sano H, Hosono A, Sumi M, Okita H, Kumamoto T, Kawai A, Hirayama J, Jyoko R, Sawada A, Nakayama H, Hosoya Y, Maeda N, Yamamoto N, Imai C, Hasegawa D, Chin M, Ozaki T. Clinical outcome of patients with recurrent or refractory localized Ewing's sarcoma family of tumors: A retrospective report from the Japan Ewing Sarcoma Study Group. Cancer Rep (Hoboken), e1329, 2021
5. Arakawa A, Ichikawa H, Kubo T, Motoi N, Kumamoto T, Nakajima M, Yonemori K, Noguchi E, Sunami K, Shiraishi K, Kakishima H, Yoshida H, Hishiki T, Kawakubo N, Kuroda T, Kiyokawa T, Yamada K, Yanaihara N, Takahashi K, Okamoto A, Hirabayashi S, Hasegawa D, Manabe A, Ono K, Matsuoka M, Arai Y, Togashi Y, Shibata T, Nishikawa H, Aoki K, Yamamoto N, Kohno T, Ogawa C. Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants. N Engl J Med, 384:42-50, 2021
6. Yoshimatsu Y, Noguchi R, Tsuchiya R, Sei A, Sugaya J, Iwata S, Sugiyama M, Yoshida A, Kawai A, Kondo T. Establishment and characterization of NCC-ssRMS1-C1: a novel patient-derived spindle-cell/sclerosing rhabdomyosarcoma cell line. Hum Cell, 33:886-893, 2020
7. Kumamoto T, Goto H, Ogawa C, Hori T, Deguchi T, Araki T, Saito AM, Manabe A, Horibe K, Toyoda H. FLEND (nelarabine, fludarabine, and etoposide) for relapsed T-cell acute lymphoblastic leukemia in children: a report from Japan Children's Cancer Group. Int J Hematol, 112:720-724, 2020
8. Nakano Y, Watanabe Y, Honda-Kitahara M, Yamagishi Y, Niizuma H, Niihori T, Sasahara Y, Sonoda Y, Narita Y, Nagane M, Kure S, Ichimura K. Utility of a bridged nucleic acid clamp for liquid biopsy: Detecting BRAF V600E in the cerebrospinal fluid of a patient with brain tumor. Pediatr Blood Cancer, 67:e28651, 2020
9. Sin Y, Yoshimatsu Y, Noguchi R, Tsuchiya R, Sei A, Ono T, Toki S, Kobayashi E, Arakawa A, Sugiyama M, Yoshida A, Kawai A, Kondo T. Establishment and characterization of a novel alveolar rhabdomyosarcoma cell line, NCC-aRMS1-C1. Hum Cell, 33:1311-1320, 2020
10. Shoji T, Kanamori M, Saito R, Watanabe Y, Watanabe M, Fujimura M, Ogawa Y, Sonoda Y, Kumabe T, Kure S, Tominaga T. Frequent Clinical and Radiological Progression of Optic Pathway/Hypothalamic Pilocytic Astrocytoma in Adolescents and Young Adults. Neurol Med Chir (Tokyo), 60:277-285, 2020
11. Horibe K, Morris JD, Tuglus CA, Dos Santos C, Kalabus J, Anderson A, Goto H, Ogawa C. A phase 1b study of blinatumomab in Japanese children with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Int J Hematol, 112:223-233, 2020
12. Hiyama E, Hishiki T, Watanabe K, Ida K, Ueda Y, Kurihara S, Yano M, Hoshino K, Yokoi A, Takama Y, Nogami Y, Taguchi T, Mori M, Kihira K, Miyazaki O, Fuji H, Honda S, Iehara T, Kazama T, Fujimura J, Tanaka Y, Inoue T, Tajiri T, Kondo S, Oue T, Yoshimura K. Outcome and Late Complications of Hepatoblastomas Treated Using the Japanese Study Group for Pediatric Liver Tumor 2 Protocol. J Clin Oncol, 38:2488-2498, 2020
13. Goto H, Yoshino Y, Ito M, Nagai J, Kumamoto T, Inukai T, Sakurai Y, Miyagawa N, Keino D, Yokosuka T, Iwasaki F, Hamanoue S, Shiomi M, Goto S. Aurora B kinase as a therapeutic target in acute lymphoblastic leukemia. Cancer Chemother Pharmacol, 85:773-783, 2020
14. Mizumoto Y, Hemmi H, Katsuda M, Miyazawa M, Kitahata Y, Miyamoto A, Nakamori M, Ojima T, Matsuda K, Nakamura M, Hayata K, Fukuda-Ohta Y, Sugiyama M, Ohta T, Orimo T, Okura S, Sasaki I, Tamada K, Yamaue H, Kaisho T. Anticancer effects of chemokine-directed antigen delivery to a cross-presenting dendritic cell subset with immune checkpoint blockade. Br J Cancer, 122:1185-1193, 2020
15. Miyazaki R, Saiga H, Kato T, Bakoshi T, Senba R, Shintani A, Suzuki M, Takao K, Sasaki I, Iizuka A, Sugiyama M, Iwami N, Fukuda-Ohta Y, Hemmi H, Tanaka T, Miyake M, Kaisho T, Hoshino K. The mechanism of action of Spi-B in the transcriptional activation of the interferon-α4 gene. Biochem Biophys Res Commun, 525:477-482, 2020
