Annual Report 2020
Innovation Center for Supportive, Palliative and Psychosocial Care
Yosuke Uchitomi, Yutaka Matsuoka, Maiko Fujimori, Sadamoto Zenda, Eriko Satomi, Masashi Kato, Ayumi Okizaki, Shinichi Goto, Tempei Miyaji, Takuhiro Yamaguchi, Miyuki Kanamaru, Maki Minemura, Asami Satake, Miyuki Kurosaki, Hiromi Hasegawa
Introduction
We have built J-SUPPORT (Japan Supportive, Palliative and Psychosocial Oncology Group) as an opened hub for multi-institutional collaborative clinical research for supportive, palliative and psychosocial care and started research management throughout Japan. (Figure 1).
In FY 2020, 23 research consultations were conducted, and a total of 17 clinical trials have been approved.
Figure 1. Organization of J-SUPPORT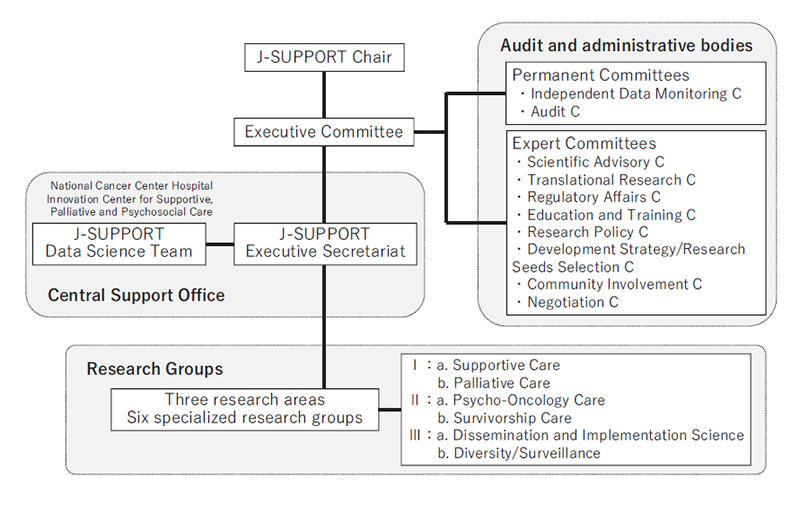

The Team and What We Do
We cooperate in providing consultation services and expert advice on clinical research design and statistical analysis to investigators as they launch new research projects in the field of supportive, palliative and psychosocial care. This service includes face-to-face or via online clinical design and biostatistics consultation, and we collaborate on studies with other study groups or institutions. We also conduct a protocol review committee and educational seminars.
Research activities
Six of the interventional trials were closed for enrollment, one was discontinued, three were in the enrollment phase, and four were in the preparation phase. One of the main articles of the approval trial was published in international journals. ‘The position statement on suicide prevention strategy in oncology’ and ‘the approaches to placebo drug settings in supportive and palliative care research’ were released.
Clinical trials
- J-SUPPORT1601Explicit prognostic disclosure to Asian women with breast cancer: A randomized, scripted video-vignette study
- J-SUPPORT1602Topical steroid versus placebo for the prevention of radiation dermatitis in head and neck cancer patients receiving chemoradiotherapy: a phase III, randomized, double-blinded trial
- J-SUPPORT1603Nurse-led, screening-triggered early specialized palliative care intervention program for advanced lung cancer patients: a randomized controlled trial
- J-SUPPORT1604A randomized, double-blind, placebo-controlled phase III trial evaluating olanzapine 5mg combined with standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy
- J-SUPPORT1605Yokukansan for perioperative psychiatric symptoms in cancer patients undergoing high invasive surgery: a randomized, double-blind, placebo-controlled trial
- J-SUPPORT1701Efficacy of hydrocolloid dressing for prophylactic use of hand-foot skin reaction induced by multi targeted kinase inhibitors: a phase III self-controlled study
- J-SUPPORT1702Quality of palliative care for end-of-life cancer patients and retrospective cohort study of unsolved clinical question of palliative care from administrative claims data
- J-SUPPORT1703Smartphone behavioral activation and problem-solving therapy for fear of recurrence among breast cancer patients: A randomized control trial
- J-SUPPORT1704Smartphone behavioral activation and problem-solving therapy for fear of recurrence among breast cancer patients: A randomized control trial
- J-SUPPORT1901A randomized Controlled trial of the Case management to Encourage participation in cancer Screening for people with Schizophrenia in psychiatric outpatient clinics
- J-SUPPORT1902Suicide, other externally caused injuries, and cardiovascular death following a cancer diagnosis: a nationwide population-based study in Japan
- J-SUPPORT1903The single-armed confirmatory trial for immediate effectivity and safety of palliative arterial embolization for painful bone metastases
- J-SUPPORT2001SMartphone Intervention to LEssen depression/Anxiety and GAIN resilience for cancer patients: SMILE AGAIN project
- J-SUPPORT2002Preoperative steroid for enhancing patients’ recovery after head and neck cancer surgery with free tissue transfer reconstruction: a phase III, placebo-controlled, randomized, double-blinded study
- J-SUPPORT2101Randomized Controlled Trial to Develop a Program for Geriatric Assessment and Management by Mobile Applications for Elderly Patients with Advanced and Recurrent Cancer
- J-SUPPORT2102Interactive assistance via eHealth for small and medium-sized enterprises' employer and health care manager teams on tobacco control
- J-SUPPORT2103Ramelteon for prevention of postoperative delirium in delirium high-risk cancer patients.: A randomized, double-blind, placebo-controlled multi-cent er trial
Education
Yosuke Uchitomi organized 'The Palliative, Supportive, and Psycho-Oncology Care Joint Meeting 2020' as the congress chairman. In addition, The D&I Science Society (Dissemination and Implementation Science Society) Scientific Meetings, Behavioral science Seminar, J-SUPPORT Seminar, and JORTC x JIVROSG x J-SUPPORT Joint Research Conference were held. Furthermore, we are promoting Patient and Public Involvement (PPI) by holding protocol review meetings and the J-SUPPORT research results debriefing meeting with patients and citizens.
Future Prospects
We will provide support in conducting J-SUPPORT-approved clinical research, facilitating the approval of new clinical trials. We will also be engaging in patient and public involvement (PPI) to promote the development infrastructure for cancer supportive care.
List of papers published in 2020
Journal
1. Wada S, Sadahiro R, Matsuoka YJ, Uchitomi Y, Yamaguchi T, Sato T, Shimada K, Yoshimoto S, Daiko H, Kanemitsu Y, Kawai A, Kato T, Fujimoto H, Shimizu K. Yokukansan for Treatment of Preoperative Anxiety and Prevention of Postoperative Delirium in Cancer Patients Undergoing Highly Invasive Surgery. J-SUPPORT 1605 (ProD Study): A Randomized, Double-Blind, Placebo-Controlled Trial. J Pain Symptom Manage, 61:71-80, 2021
2. Okamura M, Fujimori M, Sato A, Uchitomi Y. Unmet supportive care needs and associated factors among young adult cancer patients in Japan. BMC Cancer, 21:17, 2021
3. Fujisawa D, Umemura S, Okizaki A, Satomi E, Yamaguchi T, Miyaji T, Mashiko T, Kobayashi N, Kinoshita H, Mori M, Morita T, Uchitomi Y, Goto K, Ohe Y, Matsumoto Y. Nurse-led, screening-triggered, early specialised palliative care intervention programme for patients with advanced lung cancer: study protocol for a multicentre randomised controlled trial. BMJ Open, 10:e037759, 2020
4. Zenda S, Ryu A, Takashima A, Arai M, Takagi Y, Miyaji T, Mashiko T, Shimizu Y, Yamazaki N, Morizane C, Yamaguchi T, Kawaguchi T, Hanai A, Uchitomi Y, Oshiba F. Hydrocolloid dressing as a prophylactic use for hand-foot skin reaction induced by multitargeted kinase inhibitors: protocol of a phase 3 randomised self-controlled study. BMJ Open, 10:e038276, 2020
5. Fujimori M, Sato A, Jinno S, Okusaka T, Yamaguchi T, Ikeda M, Ueno M, Ozaka M, Takayama Y, Miyaji T, Majima Y, Uchitomi Y. Integrated communication support program for oncologists, caregivers and patients with rapidly progressing advanced cancer to promote patient-centered communication: J-SUPPORT 1904 study protocol for a randomised controlled trial. BMJ Open, 10:e036745, 2020
6. Harashima S, Fujimori M. Risk of suicide among adolescents and young adults with cancer and a need for targeted interventions. Ann Transl Med, 8:428, 2020
7. Matsuda Y, Tanimukai H, Inoue S, Inada S, Sugano K, Hasuo H, Yoshimura M, Wada S, Dotani C, Adachi H, Okamoto Y, Takeuchi M, Fujisawa D, Kako J, Sasaki C, Kishi Y, Akizuki N, Inagaki M, Uchitomi Y, Matsushima E, Okuyama T. JPOS/JASCC clinical guidelines for delirium in adult cancer patients: a summary of recommendation statements. Jpn J Clin Oncol, 50:586-593, 2020
8. Chen SY, Fujimori M, Wang HM, Tang WR. Gender Differences in Cancer Patients' Preferences for Truth-Telling in Taiwan. Cancer Nurs, 2020
9. Higuchi Y, Fujiwara M, Nakaya N, Fujimori M, Yamada Y, Wada R, Etoh T, Kakeda K, Uchitomi Y, Yamada N, Inagaki M. Trends in smoking rates among individuals with serious psychological distress: Analysis of data from a Japanese national survey, 2007-2016. Psychiatry Res, 291:113225, 2020
10. Mollica MA, Mayer DK, Oeffinger KC, Kim Y, Buckenmaier SS, Sivaram S, Muha C, Taib NA, Andritsch E, Asuzu CC, Bochis OV, Diaz S, Trill MD, Garcia PJ, Grassi L, Uchitomi Y, Shaikh AJ, Jefford M, Lee HJ, Johansen C, Luyirika E, Maher EJ, Mallillin MMB, Maniragaba T, Mehnert-Theuerkauf A, Pramesh CS, Siesling S, Spira O, Sussman J, Tang L, Hai NV, Yalcin S, Jacobsen PB . Follow-Up Care for Breast and Colorectal Cancer Across the Globe: Survey Findings From 27 Countries. JCO Glob Oncol, 6:1394-1411, 2020
11. Oshima E, Takenoshita S, Iwai R, Yabe M, Imai N, Horiuchi M, Takeda N, Uchitomi Y, Yamada N, Terada S . Competency of aMCI patients to consent to cholinesterase treatment. Int Psychogeriatr, 32:211-216, 2020
12. Fujiwara M, Higuchi Y, Nakaya N, Fujimori M, Yamada Y, Wada R, Etoh T, Kakeda K, Uchitomi Y, Nakayama T, Yamada N, Inagaki M. Trends in cancer screening rates among individuals with serious psychological distress: an analysis of data from 2007 to 2016 Japanese national surveys. Journal of Psychosocial Oncology Research & Practice, 2:e205, 2020
