Annual Report 2020
Department of Cellular Therapy Processing
Takahiro Fukuda, Minoru Kojima
Introduction
The Department of Cellular Therapy Processing was established in 2020 as a shared department at NCCH to handle appropriately the increasing need for cellular therapy in recent years. We support cell collection, preparation, and storage not only for hematopoietic stem cell transplantation (HSCT) but also for new cellular therapies such as Chimeric Antigen Receptor T-cell (CAR-T) therapy.
The Team and What We Do
Our department consists of 2 physicians, 10 clinical engineers, and 5 medical technicians. In fiscal 2020, the number of cell collections was 28 for autologous peripheral blood stem cells (PBSCs), 35 for related PBSCs, 8 for unrelated PBSCs, and 8 for unrelated bone marrow from Japan Marrow Donor Program (JDMP) donors (Table 1). Of these, 63 cell collections were cryopreserved. We annually performed 29 related peripheral blood stem cell transplantations (PBSCTs) (including 20 HLA-haploidentical), 20 for unrelated PBSCTs, 7 for unrelated bone marrow transplantations (BMTs), 15 for cord blood transplantations (CBTs), and 24 for autologous PBSCTs. The COVID-19 pandemic caused the delay of coordination through the JMDP, but the use of HLA-haploidentical transplantation and CBT compensated for the delay and contributed to maintaining the number of transplantations.
For CAR-T therapy, it is essential to establish a strict management system, good manufacturing practice (GMP), for cell collection and processing. We have constructed the GMP system and our center was certified as a treatment facility of Kymriah®. The number of autologous lymphocyte collections for CAR-T therapy was 11 in clinical trials and 14 in clinical practice, and the final CAR-T product was infused in 9 patients in clinical trials and 6 patients in clinical practice (Table 1).
Research activities
In the annual meeting of the Japanese Society of Hematopoietic Cell Transplantation, we presented two retrospective single-center studies of mononuclear cell separation in unrelated bone marrow transplantations (Fukumoto, JSHCT 2021, Nakabayashi, JSHCT 2021). We have published a single-center retrospective study that demonstrated the usefulness of hematopoietic progenitor cell (HPC) monitoring to predict autologous PBSC harvest (Kasane, TAS 2021).
Table 1. Number of each type of procedures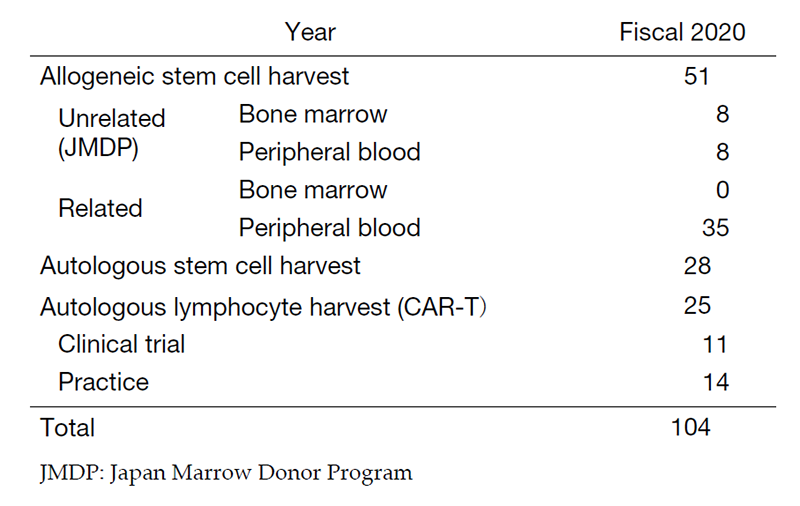

Clinical trials
We are involved in clinical trials of CAR-T therapies. 2 clinical trials in the Department of Experimental Therapeutics (TAK-102) and 9 clinical trials in the Department of Hematology (JCAR017 BCM-001, JCAR017 BCM-003 and JCAR017 FOL-001) were conducted.
Education
We have trained 2 clinical engineers to develop their skills for blood separator operation and 1 medical technician to develop her skills for cellular preparation in fiscal 2020.
Future Prospects
In recent years, the number of allogeneic PBSCTs has increased due to the expansion of HLA-haploidentical hematopoietic stem cell transplantation. In addition, it has been reported that the number of CAR-T therapy is increasing in the United States. In Japan, Breanji® (BMS) and Yescarta® (Daiichi Sankyo) will be approved as new CAR-T therapies in 2021. This tendency is expected to accelerate. It is anticipated that the role of this department will increase in the future, and we need to expand our system to provide appropriate treatment for all patients.
