Annual Report 2020
Department of Biobank and Tissue Resources
Yasushi Yatabe, Nobuyoshi Hiraoka, Hiromi Sakamoto, Koya Shiraishi, Masumi Tanaka, Teiko Yamane, Izumi Hayama, Yuka Izumi, Takako Sakamoto
Introduction
Since 2002, the NCC Biobank has been working to build and operate a biobank with a catalog database containing high-quality, standardized clinical and pathological information.
The Team and What We Do
The collection status of the blood and tissue samples in the past three years is summarized in Table 1.
The lower number of samples collected in FY2020 was due to the suspension of blood and tissue collection services from April 14, 2020, following the spread of COVID-19 and the declaration of a state of emergency by the government. Particularly, outpatient clinic for new patients were suspended from March 31 to April 14, 2020 due to nosocomial infections. Specimen collection dropped significantly in April and May, but recovered to the same level as January 2020 in September. The monthly collection status is shown in Figure 1.
Table 1. Status of sample collection for the past three years 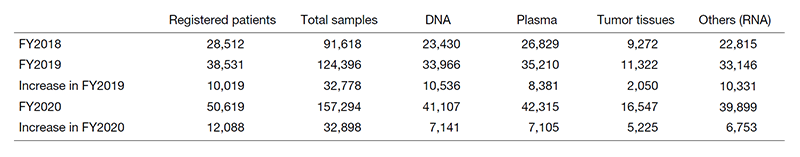

Figure 1. Changes in the sample collection by month in FY2020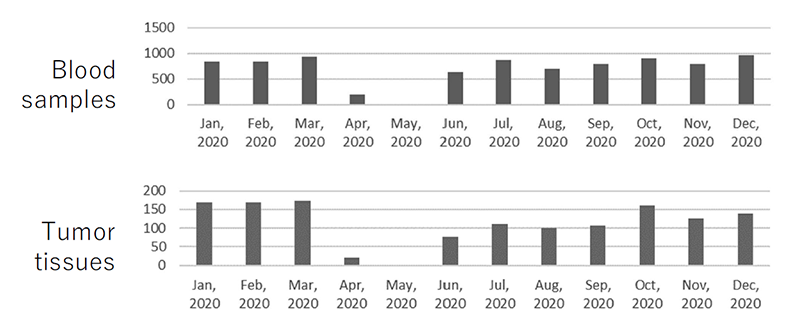

Table 2. Contribution to publishing articles using NCC Biobank samples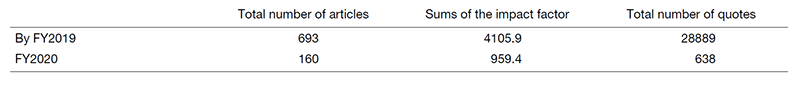

Research activities
The total number of articles published in FY2020 on research using biobank samples was high, with 113 papers and a total impact factor of 670.1 (average 6.38, highest 42.8), suggesting that the biobank has contributed sufficiently to the promotion of translational researches so far (Table 2).
Education
We accepted visits and interviews from biobank staff and practitioners from other institutions. In addition, we were actively involved in the management of the National Center Biobank Network (NCBN), and cooperated with Tohoku Megabank and BBJ to build a cross-sectional search system.
Future Prospects
Some problems have been pointed out in promoting the sample collection that meet research needs and the provision of samples that combine clinical information. The temperature control system was introduced this year, and issues related to the sample distribution and confirmation of the consistency of the catalog database will be discussed in the next year.
