Annual Report 2020
Tsuruoka Metabolomics Laboratory (Team Makinoshima)
Hideki MAKINOSHIMA (Team Leader), Joji NAKAYAMA (Visiting Scientist), Yuka NOZAKI (Visiting Scientist) and Ami MARUYAMA (Visiting Scientist).
Introduction
At the time of carcinogenesis or cancer progression, homeostasis that keeps the state of the living body constant is broken. We hypothesized that metabolites that induce carcinogenesis and the metabolic system unique to cancer cells are overproduced or reduced in the individual, and the metabolites themselves contribute to the development and malignancy of cancer beyond its homeostatic mechanism. We are analyzing cancer cells, tumor tissues and blood samples from the viewpoint of cancer metabolism.
The Team and What We Do
In fiscal 2020 as well, we aimed to identify metabolites and metabolic pathways peculiar to cancer and to discover new drugs targeting them. In collaborated researches between the Institute for Advanced Biosciences, Keio University and the National Cancer Center, samples were collected from patient-derived samples, cancer cell lines, and mouse models, and metabolome analysis on cancer was performed. In drug discovery research targeting the metabolic pathways characteristic of cancer, we focused on revealing the regulation mechanisms for nucleic acids metabolism (Figure 1). We proposed the possibility of a new therapeutic method that inhibits the novel biosynthetic pathway of purine nucleic acid with antifolates and the reuse pathway with a new drug. We elucidated the nucleic acid metabolism control mechanism of small cell lung cancer, analyzed cholesterol metabolites related to breast cancer metastasis, elucidated control mechanism of nucleic acid biosynthesis in breast cancer, and clarified the mechanism of drug resistance to antifolate antagonists in malignant pleural mesenteric tumors.
Figure 1. Nucleic acid metabolism 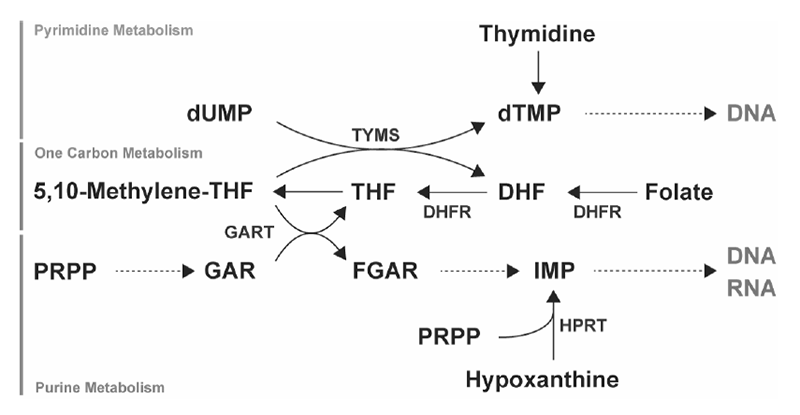

Research activities
We established several cell lines in which the gene of the enzyme HPRT1 important for the purine nucleic acid reuse pathway was deleted from genome, investigated the susceptibility to antifolates, and performed new biosynthesis and re-growth. We examined the possibility of combination therapy using inhibitors of the utilization route. Part of the molecular mechanisms that control cancer cell metastasis and gastrula invasion are shared; we performed screening for compounds that interfere with gastrula invagination in zebrafish embryos, and inhibiting metabolic enzymes involved in cortisol production, which was the HSD11β1 Inhibitor adrenosterone, was identified. Treatment of breast cancer cells with this adrenosterone can suppress the motility of breast cancer cells, and also in an experimental system in which human breast cancer cells were transplanted into zebrafish, we obtained the results in which adrenosterone suppresses metastasis. These results were published as a paper in an American cancer journal. HPRT1-deficient cells were established in breast cancer and malignant pleural mesothelioma. Furthermore, in malignant pleural mesothelioma, drug-resistant cell lines of antifolates were established.
Education
Yuki KONNO (Keio University Institute for Advanced Biosciences, Special Research Student)
Naoto Osu (Gunma University Graduate School of Medicine, Internship)
Future Prospects
In fiscal 2021, we will continue the characterization of HPRT1-deficient cells and antifolate-resistant cells to elucidate the nucleic acid biosynthesis control mechanism. At the same time, we are studying the control mechanism of nucleic acid metabolic pathways using animal models, and we plan to continue to verify it from next year onward.
List of papers published in 2020
Journal
1. Zebrafish-Based Screening Models for the Identification of Anti-Metastatic Drugs.
https://pubmed.ncbi.nlm.nih.gov/32455810/
Nakayama J, Makinoshima H.Molecules. 2020 May 21;25(10):2407. doi: 10.3390/molecules25102407.
PMID: 32455810
2. Uptake of collagen type I via macropinocytosis cause mTOR activation and anti-cancer drug resistance.
https://pubmed.ncbi.nlm.nih.gov/32201076/
Yamazaki S, Su Y, Maruyama A, Makinoshima H, Suzuki J, Tsuboi M, Goto K, Ochiai A, Ishii G.Biochem Biophys Res Commun. 2020 May 21;526(1):191-198. doi: 10.1016/j.bbrc.2020.03.067. PMID: 32201076
