Annual Report 2021
Department of Gastrointestinal Medical Oncology
Ken Kato, Atsuo Takashima, Satoru Iwasa, Yoshitaka Honma, Hirokazu Shoji, Natsuko Okita, Hidekazu Hirano, Shun Yamamoto
Introduction
The Department of Gastrointestinal Medical Oncology focuses on treatment in clinical practice, development of new drugs, and establishment of standard chemotherapy regimens including multi-modality treatment with surgery and/or radiotherapy for gastric/colorectal/gastrointestinal stromal tumor (GIST), and other gastrointestinal (GI) malignancies.
The Team and What We Do
The staff of our division consists of 7.5 medical oncologists and three or four rotating residents. We have a daily case conference every evening for discussing each patient’s treatment and a weekly research conference for sharing and discussing the progress of clinical studies and/or translational research. Multi-disciplinary team meetings with the surgical divisions (Colorectal and Gastric Surgery Divisions) and the Radiation Oncology Division are held weekly to decide optimal treatment strategies for each patient and to discuss the treatment consensus for each disease. From April 2021 to March 2022, we treated 527 hospitalized patients (excluding head and neck and esophageal departments, and including 216 new patients), including 223 with gastric cancer, 237 with colorectal cancer, 17 with anal canal cancer, 10 with GIST, and 9 with small bowel cancer.
Research activities
clinical research to establish new treatments and answer clinical questions. As for the late phase clinical studies, our division is playing a leading role in JCOG (Japan Clinical Oncology Group) and WJOG (West Japan Oncology Group), which are the largest cooperative study groups for cancer treatment in Japan. As an early phase clinical study, in collaboration with other active hospitals, we are conducting investigator-initiated trials using unapproved drugs under the regulation of the GCP (Good Clinical Practice). Moreover, we collaborate with the National Cancer Center Research Institute and other distinguished basic research institutions to conduct clinical research based on our own original new ideas and findings. From March 2021 to March 2022, 147 patients were enrolled in clinical trials/trials (Table 1). We presented in 46 conferences, 19 of which were international. We published 58 papers, 45 of which were original article in English, 8 review article in English, and 5 in Japanese. Of these, our department was the First or Corresponding Author for 20, and most of them were international multicenter trials, investigator-initiated trials, JCOG trials, and their accompanying results.
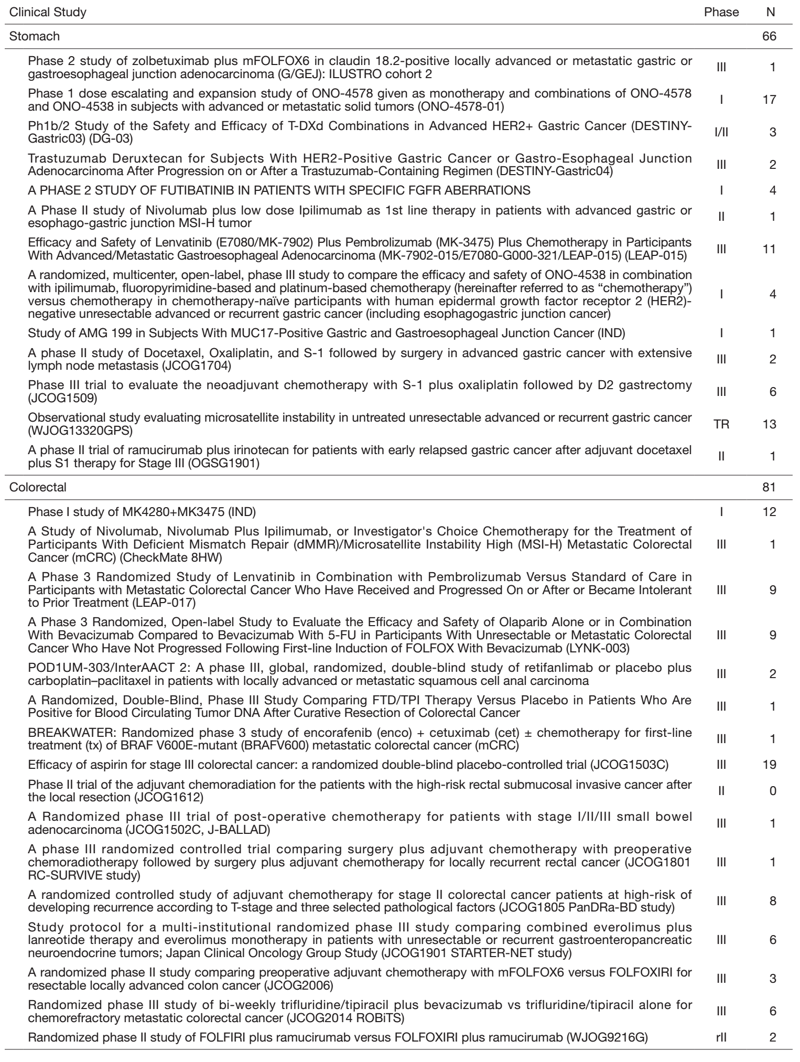
Clinical trials
We are conducting many clinical trials, including JCOG, WJOG, company-initiated, and other collaborative investigator-initiated trials and in-house clinical trials. A total of 147 patients were enrolled in 2021 (Table 1).
Education
In clinical practice, full-time doctors and oncology trainees/residents form teams and conduct guidance through the patients in their charge. In addition, we assign research themes to oncology trainees/residents, and energetically provide guidance on clinical research planning, management, conference presentations, and thesis writing. From April 2021 to March 2022, 10 English papers and 5 Japanese papers were written by cancer specialists/residents of our department as the first author.
Future Prospects
Our division focus on “clinical practice”, “education” and “clinical research” for the development of gastrointestinal cancer treatment. For daily practice, we give the patients the standard treatment; moreover, we will always pay attention to improving patient care to satisfy unmet clinical needs. For education, we will keep educating the residents and chief residents; moreover the staff doctors will strive to keep improving themselves. For clinical research, we will strive toward the next step of clinical development to establish new global standard treatments.
List of papers published in 2021
Journal
1. Oshima K, Kato K, Ito Y, Daiko H, Nozaki I, Nakagawa S, Shibuya Y, Kojima T, Toh Y, Okada M, Hironaka S, Akiyama Y, Komatsu Y, Maejima K, Nakagawa H, Onuki R, Nagai M, Kato M, Kanato K, Kuchiba A, Nakamura K, Kitagawa Y. Prognostic biomarker study in patients with clinical stage I esophageal squamous cell carcinoma: JCOG0502-A1. Cancer science, 113:1018-1027, 2022
2. Kikuchi K, Yamazaki N, Nozawa K, Fukuda H, Shibata T, Machida R, Hamaguchi T, Takashima A, Shoji H, Boku N, Takatsuka S, Takenouchi T, Nishina T, Yoshikawa S, Takahashi M, Hasegawa A, Kawazoe A, Masuishi T, Mizutani H, Kiyohara Y. Topical corticosteroid therapy for facial acneiform eruption due to EGFR inhibitors in metastatic colorectal cancer patients: a randomized controlled trial comparing starting with a very strong or a weak topical corticosteroid (FAEISS study, NCCH1512, colorectal part). Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer, 30:4497-4504, 2022
3. Takemura C, Kashima J, Hashimoto T, Ichikawa H, Honma Y, Goto Y, Watanabe SI, Yatabe Y. A mimic of lung adenocarcinoma: a case report of histological conversion of metastatic thyroid papillary carcinoma. Histopathology, 80:1004-1007, 2022
4. keda G, Yamamoto S, Kato K. The safety of current treatment options for advanced esophageal cancer after first-line chemotherapy. Expert opinion on drug safety, 21:55-65, 2022
5. Muro K, Kojima T, Moriwaki T, Kato K, Nagashima F, Kawakami H, Ishihara R, Ogata T, Satoh T, Iwakami K, Han S, Yatsuzuka N, Takami T, Bhagia P, Doi T. Second-line pembrolizumab versus chemotherapy in Japanese patients with advanced esophageal cancer: subgroup analysis from KEYNOTE-181. Esophagus: official journal of the Japan Esophageal Society, 19:137-145, 2022
6. Shibayama T, Shimoi T, Mori T, Noguchi E, Honma Y, Hijioka S, Yoshida M, Ogawa C, Yonemori K, Yatabe Y, Yoshida A. Cytokeratin-positive Malignant Tumor in the Abdomen With EWSR1/FUS-CREB Fusion: A Clinicopathologic Study of 8 Cases. The American journal of surgical pathology, 46:134-146, 2022
7. Mori Y, Kikuchi O, Horimatsu T, Hara H, Hironaka S, Kojima T, Kato K, Tsushima T, Ishihara R, Mukai K, Uozumi R, Tada H, Kasai H, Kawaguchi A, Muto M. Multicenter phase II study of trifluridine/tipiracil for esophageal squamous carcinoma refractory/intolerant to 5-fluorouracil, platinum compounds, and taxanes: the ECTAS study. Esophagus: official journal of the Japan Esophageal Society, 19:444-451, 2022
8. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. The New England journal of medicine, 386:449-462, 2022
9. Yoshikawa AK, Yamaguchi K, Muro K, Takashima A, Ichimura T, Sakai D, Kadowaki S, Chin K, Kudo T, Mitani S, Kitano S, Thai D, Zavodovskaya M, Liu J, Boku N, Satoh T. Safety and tolerability of andecaliximab as monotherapy and in combination with an anti-PD-1 antibody in Japanese patients with gastric or gastroesophageal junction adenocarcinoma: a phase 1b study. Journal for immunotherapy of cancer, 10:2022
10. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. The Lancet. Oncology, 23:234-247, 2022
11. Kato K, Ito Y, Nozaki I, Daiko H, Kojima T, Yano M, Ueno M, Nakagawa S, Takagi M, Tsunoda S, Abe T, Nakamura T, Okada M, Toh Y, Shibuya Y, Yamamoto S, Katayama H, Nakamura K, Kitagawa Y. Parallel-Group Controlled Trial of Surgery Versus Chemoradiotherapy in Patients With Stage I Esophageal Squamous Cell Carcinoma. Gastroenterology, 161:1878-1886.e2, 2021
12. Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Nishiyama T, Chen LT, Kang YK. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24:946-958, 2021
13. Shida D, Boku N, Nakamura Y, Yoshida T, Tanabe T, Yasui K, Takashima A, Kanemitsu Y. Comparison of model fit and discriminatory ability of M category as defined by the 7th and 8th editions of the tumor-node-metastasis classification of colorectal cancer and the 9th edition of the Japanese classification. Cancer medicine, 10:6937-6946, 2021
14. Tanabe T, Shida D, Boku N, Yoshida T, Tsukamoto S, Takashima A, Kanemitsu Y. Primary Tumor-Related Complications Among Patients With Unresectable Stage IV Colorectal Cancer in the Era of Targeted Therapy: A Competing Risk Regression Analysis. Diseases of the colon and rectum, 64:1074-1082, 2021
15. Nakamura Y, Shida D, Boku N, Yoshida T, Tanabe T, Takamizawa Y, Takashima A, Kanemitsu Y. Lymphocyte-to-C-Reactive Protein Ratio Is the Most Sensitive Inflammation-Based Prognostic Score in Patients With Unresectable Metastatic Colorectal Cancer. Diseases of the colon and rectum, 64:1331-1341, 2021
16. Ueno H, Kajiwara Y, Ajioka Y, Sugai T, Sekine S, Ishiguro M, Takashima A, Kanemitsu Y. Histopathological atlas of desmoplastic reaction characterization in colorectal cancer. Japanese journal of clinical oncology, 51:1004-1012, 2021
17. akahashi S, Sakamoto Y, Denda T, Takashima A, Komatsu Y, Nakamura M, Ohori H, Yamaguchi T, Kobayashi Y, Baba H, Kotake M, Amagai K, Kondo H, Shimada K, Sato A, Yuki S, Okita A, Ouchi K, Komine K, Watanabe M, Morita S, Ishioka C. Advanced colorectal cancer subtypes (aCRCS) help select oxaliplatin-based or irinotecan-based therapy for colorectal cancer. Cancer science, 112:1567-1578, 2021
18. Takamizawa S, Honma Y, Murakami N, Mori T, Oka H, Yamamoto S, Kashihara T, Ito K, Kubo Y, Ikeda A, Matsumoto F, Omura G, Kobayashi K, Itami J, Kato K, Yoshimoto S. Short-term outcomes of induction chemotherapy with docetaxel, cisplatin, and fluorouracil (TPF) in locally advanced nasopharyngeal carcinoma. Investigational new drugs, 39:564-570, 2021
19. Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, Mamon HJ, Moehler M, Fu X, Cho BC, Bordia S, Bhagia P, Shih CS, Desai A, Enzinger P. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future oncology (London, England), 17:1143-1153, 2021
20. Ota Y, Noguchi T, Ariji E, Fushimi C, Fuwa N, Harada H, Hayashi T, Hayashi R, Honma Y, Miura M, Mori T, Nagatsuka H, Okura M, Ueda M, Uzawa N, Yagihara K, Yagishita H, Yamashiro M, Yanamoto S, Kirita T. General rules for clinical and pathological studies on oral cancer (2nd edition): a synopsis. International journal of clinical oncology, 26:623-635, 2021
21. Kashihara T, Nakamura S, Murakami N, Ito K, Matsumoto Y, Kobayashi K, Omura G, Mori T, Honma Y, Kubo Y, Okamoto H, Takahashi K, Inaba K, Okuma K, Igaki H, Nakayama Y, Kato K, Matsumoto F, Yoshimoto S, Itami J. Initial Experience of Intentional Internal High-Dose Policy Volumetric Modulated Arc Therapy of Neck Lymph Node Metastases ≥ 2 cm in Patients With Head and Neck Squamous Cell Carcinoma. Frontiers in oncology, 11:651409, 2021
22. Aoki M, Iwasa S, Boku N. Trastuzumab deruxtecan for the treatment of HER2-positive advanced gastric cancer: a clinical perspective. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24:567-576, 2021
23. Mikuni H, Yamamoto S, Kato K. Nivolumab for the treatment of esophageal cancer. Expert opinion on biological therapy, 21:697-703, 2021
24. Kosaka M, Honma Y, Ishiki H. Unresolved questions regarding the promise of the TPEx regimen. The Lancet. Oncology, 22:e227, 2021
25. Chamseddine AN, Oba K, Buyse M, Boku N, Bouché O, Satar T, Auperin A, Paoletti X . Impact of follow-up on generalized pairwise comparisons for estimating the irinotecan benefit in advanced/metastatic gastric cancer. Contemporary clinical trials, 105:106400, 2021
26. Miura Y, Ando M, Yamazaki K, Hironaka S, Boku N, Muro K, Hyodo I. Time-dependent discrepancies between physician-assessed and patient-reported oxaliplatin-induced peripheral neuropathy in patients with metastatic colorectal cancer who received mFOLFOX6 plus bevacizumab: a post hoc analysis (WJOG4407GSS2). Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer, 29:3715-3723, 2021
27. Tsutsui K, Kikuchi K, Nozawa K, Takashima A, Tsuchiyama K, Namikawa K, Aiba S, Yamazaki N. Efficacy and safety of topical benzoyl peroxide for prolonged acneiform eruptions induced by cetuximab and panitumumab: A multicenter, phase II trial. The Journal of dermatology, 48:1077-1080, 2021
28. Wada Y, Shimada M, Murano T, Takamaru H, Morine Y, Ikemoto T, Saito Y, Balaguer F, Bujanda L, Pellise M, Kato K, Saito Y, Ikematsu H, Goel A. A Liquid Biopsy Assay for Noninvasive Identification of Lymph Node Metastases in T1 Colorectal Cancer. Gastroenterology, 161:151-162.e1, 2021
29. Yamamoto S, Nagashima K, Kawakami T, Mitani S, Komoda M, Tsuji Y, Izawa N, Kawakami K, Yamamoto Y, Makiyama A, Yamazaki K, Masuishi T, Esaki T, Nakajima TE, Okuda H, Moriwaki T, Boku N. Second-line chemotherapy after early disease progression during first-line chemotherapy containing bevacizumab for patients with metastatic colorectal cancer. BMC cancer, 21:1159, 2021
30. Satoh T, Kato K, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Doki Y, Matsumura Y, Kitagawa Y. Five-year follow-up of nivolumab treatment in Japanese patients with esophageal squamous-cell carcinoma (ATTRACTION-1/ONO-4538-07). Esophagus: official journal of the Japan Esophageal Society, 18:835-843, 2021
31. Yamaguchi T, Takashima A, Nagashima K, Terashima M, Aizawa M, Ohashi M, Tanaka R, Yamada T, Kinoshita T, Matsushita H, Ishiyama K, Hosoda K, Yuasa Y, Haruta S, Kakihara N, Nishikawa K, Yunome G, Satoh T, Fukagawa T, Katai H, Boku N. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): a multi-institutional retrospective study. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24:701-709, 2021
32. Hirose T, Yamamoto S, Kato K. Emerging data on nivolumab for esophageal squamous cell carcinoma. Expert review of gastroenterology & hepatology, 15:845-854, 2021
33. .Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (London, England), 398:759-771, 2021
34. Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, Yuki S, Masuishi T, Nishina T, Ebi H, Sawada K, Taniguchi H, Fuse N, Nomura S, Fukui M, Matsuda S, Sakamoto Y, Uchigata H, Kitajima K, Kuramoto N, Asakawa T, Olsen S, Odegaard JI, Sato A, Fujii S, Ohtsu A, Yoshino T. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nature medicine, 27:1899-1903, 2021
35. Ishikawa M, Takashima A, Nagata Y, Sawada R, Aoki M, Imazeki H, Hirano H, Shoji H, Honma Y, Iwasa S, Okita N, Kato K, Saruta M, Boku N. Tumor growth rate during re-challenge chemotherapy with previously used agents as salvage treatment for metastatic colorectal cancer: A retrospective study. PloS one, 16:e0257551, 2021
36. Denda T, Takashima A, Gamoh M, Iwanaga I, Komatsu Y, Takahashi M, Nakamura M, Ohori H, Sakashita A, Tsuda M, Kobayashi Y, Baba H, Kotake M, Ishioka C, Yamada Y, Sato A, Yuki S, Morita S, Takahashi S, Yamaguchi T, Shimada K. Combination therapy of bevacizumab with either S-1 and irinotecan or mFOLFOX6/CapeOX as first-line treatment of metastatic colorectal cancer (TRICOLORE): Exploratory analysis of RAS status and primary tumour location in a randomised, open-label, phase III, non-inferiority trial. European journal of cancer (Oxford, England: 1990), 154:296-306, 2021
37. Nakajima H, Fukuoka S, Masuishi T, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Negoro Y, Komoda M, Makiyama A, Denda T, Hatachi Y, Suto T, Sugimoto N, Enomoto M, Ishikawa T, Kashiwada T, Ando K, Yuki S, Okuyama H, Kusaba H, Sakai D, Okamoto K, Tamura T, Yamashita K, Gosho M, Moriwaki T. Clinical Impact of Primary Tumor Location in Metastatic Colorectal Cancer Patients Under Later-Line Regorafenib or Trifluridine/Tipiracil Treatment. Frontiers in oncology, 11:688709, 2021
38. Niisato Y, Moriwaki T, Fukuoka S, Masuishi T, Takashima A, Kumekawa Y, Kajiwara T, Yamazaki K, Esaki T, Makiyama A, Denda T, Hatachi Y, Suto T, Sugimoto N, Shimada Y. Clinical Outcomes Following Trifluridine/Tipiracil Treatment for Patients With Metastatic Colorectal Cancer Ineligible for Regorafenib Treatment. Anticancer research, 41:2203-2207, 2021
39. Miyamoto T, Kato K. Immunotherapy for esophageal carcinoma: a narrative review. Shanghai Chest, 5:2021
40. Ego M, Abe S, Nakatani Y, Nonaka S, Suzuki H, Yoshinaga S, Oda I, Kato K, Honma Y, Itami J, Daiko H, Saito Y, Boku N. Long-term outcomes of patients with recurrent squamous cell carcinoma of the esophagus undergoing salvage endoscopic resection after definitive chemoradiotherapy. Surgical endoscopy, 35:1766-1776, 2021
41. Yagishita S, Kato K, Takahashi M, Imai T, Yatabe Y, Kuwata T, Suzuki M, Ochiai A, Ohtsu A, Shimada K, Nishida T, Hamada A, Mano H. Characterization of the large-scale Japanese patient-derived xenograft (J-PDX) library. Cancer science, 112:2454-2466, 2021
42. Abe S, Matsuzaki J, Sudo K, Oda I, Katai H, Kato K, Takizawa S, Sakamoto H, Takeshita F, Niida S, Saito Y, Ochiya T. A novel combination of serum microRNAs for the detection of early gastric cancer. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association, 24:835-843, 2021
43. Ito T, Fujimori N, Honma Y, Kudo A, Hijioka S, Katsushima S, Kimura Y, Fukutomi A, Hisamatsu S, Nakajima A, Shimatsu A. Long-term safety and efficacy of lanreotide autogel in Japanese patients with neuroendocrine tumors: Final results of a phase II open-label extension study. Asia-Pacific journal of clinical oncology, 17:e153-e161, 2021
