Annual Report 2021
Department of Pharmacy
Tetsuya Furukawai
Introduction
The Department of Pharmacy stores and dispenses drugs, prepares injections (including aseptic mixtures), collects and disseminates drug information, and provides patients with guidance regarding the proper use of drugs. Its services have been improved toward the hospital’s goal of achieving the highest quality of medical care, practice, and research. A state-of-the-art computerized system and other pharmacy-related equipment ensure quality control and inventory management, promote the proper use of drugs, and enhance the efficiency and quality of our services.
The Team and What We Do
As part of the fundamental function of the hospital, the Department of Pharmacy prepares and dispenses oral and topical medicines and injections for individual patients. All outpatients and inpatients are provided with aseptic mixtures of injectable chemotherapy agents prepared in our department. As the importance of providing drug information for patients has been widely acknowledged, clinical pharmacists visit inpatients and give advice on taking medicine, focusing especially on pain control with opioids, and participate in the palliative-care support team, while our department provides outpatients with guidance on the proper use of opioids and anti-cancer drugs. Our department also places pharmacists in every hospital ward to provide medication reconciliation services for inpatients, with a view to enhancing the quality of chemotherapy as well as to ease burdens on doctors and nurses. Pharmacists collect, compile, and maintain a database of drug information and distribute pertinent information to the medical staff. Drug information is disseminated quickly throughout the hospital by paper distribution and/or on the in-hospital computer network. Pharmacists individualize dosage regimens for specified drugs such as tacrolimus, aminoglycosides, and vancomycin based on both measured blood concentrations and pharmacokinetic analysis to maximize their efficacy, and to minimize adverse events. A physician places an order through the hospital’s computerized electric medical record system. The prescription order is then redirected to the medicine-package-printing system which provides drug information. The medicine-package information, instructions and explanations (which are easy to understand for patients so as to ensure the proper use of drugs, and include matters such as efficacy and effectiveness, precautions, and guidance concerning symptoms in the early stage of adverse reactions) are automatically printed out for patients when a prescription is ordered. The injection order is directly linked to an automatic “picking system” device, and this linkage ensures that injections are made properly and efficiently. This injection-ordering system contains an additional function which is a regimen-ordering system for anti-cancer drugs that makes it possible to check the dose as well as the interval of chemotherapy. The Department of Pharmacy also has a robot which prepares injection preparations without human assistance.
Table 1. Number of Prescriptions in 2021

Table 2. Amounts of Drug Consumed in 2021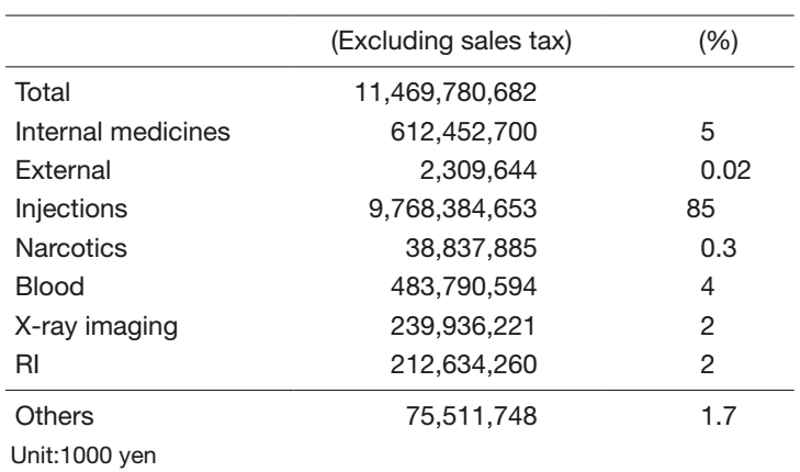

Table 3. Aseptic Preparation of Injectable Drugs in 2021

Table 4. Investigational Drugs

Research activities
Since an important mission of the Department of Pharmacy is to contribute to the development of new drugs, inventory control and handling of new investigative drugs are performed in accordance with Good Clinical Practice regulations. Research on the safety management of chemotherapy is conducted including handling of chemotherapeutic drugs, reduction of incidents regarding drugs, and improvement of pain control for patients who need palliative care through the use of guidance materials. A couple of studies on the pharmacokinetics and pharmacodynamics of cancer-related drugs have been performed and some of the results have been reported in international conferences and journals.
Clinical trials
We participated in a clinical trial about chemotherapy-induced nausea and vomiting. We developed a new antiemetic therapy which combines olanzapine (atypical antipsychotic) with standard antiemetic therapy for highly emetogenic chemotherapy for breast cancer patients. A protocol paper for this trial was published in 2021.
Education
The National Cancer Center Hospital offers a three-year postgraduate pharmacy residency training in clinical oncology. In the first year, the program places the most importance on the technical aspects of cancer care. In the second year, through required rotations in a variety of focused hematology/oncology services, residents will refine their clinical problem-solving skills in cancer management and patient education, as well as provide pharmaceutical care to ambulatory care patients and participate in an oncology-focused Drug Information Program. In the third year, residents participate in specialized pharmaco-clinical practice and research activities, which may be tailored to their goals. The hospital also provides a two-year chief residency program in which post-residency trainees may develop their clinical research capabilities to a higher level. Moreover, there are opportunities for educational activities, such as a training course for visiting expert pharmacists and post-graduate students of pharmacy, and participation in a multi-institutional TV conference.
Future Prospects
In order to provide the best cancer care, the Department of Pharmacy promotes “the best cancer treatments”, “developing and spreading of new treatments”, “educating young pharmacists for oncology”, and “providing information to domestic and overseas organizations”.
List of papers published in 2021
Journal
1. Abe K, Maeda-Minami A, Ishizu T, Iwata S, Kobayashi E, Shimoi T, Kawano Y, Hashimoto H, Yamaguchi M, Furukawa T, Miyazaki S, Mano Y. Risk Factors for Hepatic Toxicity of High-dose Methotrexate in Patients With Osteosarcoma. Anticancer research, 42:1043-1050, 2022
2. Nakashima T, Inamoto Y, Aoki J, Ito A, Tanaka T, Kim SW, Hashimoto H, Fukuda T, Furukawa T. Differences in kinetics of tacrolimus concentration after letermovir discontinuation by type of concomitant azole antifungal. International journal of hematology, 115:158-162, 2022
3. Saito Y, Kumamoto T, Yamaguchi M, Ogawa C, Kato M. Use of Pegfilgrastim in Japanese Pediatric Patients With Solid Tumors: A Retrospective Analysis. Journal of pediatric hematology/oncology, 44:e386-e390, 2022
4. Hirayama T, Kojima R, Udagawa R, Yanai Y, Ogawa Y, Shindo A, Tanaka M, Kobayashi M, Ishiki H, Satomi E. A Questionnaire Survey on Adolescent and Young Adult Hiroba, a Peer Support System for Adolescent and Young Adult Cancer Patients at a Designated Cancer Center in Japan. Journal of adolescent and young adult oncology, 11:309-315, 2022
5. Hirayama T, Kojima R, Udagawa R, Yanai Y, Ogawa Y, Tanaka M, Kayano A, Mashiko Y, Ogata K, Ishiki H, Satomi E. A Hospital-Based Online Patients Support Program, Online Adolescent and Young Adult Hiroba, for Adolescent and Young Adult Cancer Patients at a Designated Cancer Center in Japan. Journal of adolescent and young adult oncology, 2022
6. Hibino H, Sakiyama N, Makino Y, Makihara-Ando R, Horinouchi H, Fujiwara Y, Kanda S, Goto Y, Yoshida T, Okuma Y, Shinno Y, Murakami S, Hashimoto H, Akiyoshi T, Imaoka A, Ohe Y, Yamaguchi M, Ohtani H. Evaluation of hepatic CYP3A enzyme activity using endogenous markers in lung cancer patients treated with cisplatin, dexamethasone, and aprepitant. European journal of clinical pharmacology, 78:613-621, 2022
7. Ozeki R, Iihara H, Shimokawa M, Hashimoto H, Abe M, Mukohara T, Bando H, Hayashi T, Kawazoe H, Komoda M, Yanai Takahashi T, Saito M. Study protocol for a double-blind, comparative, randomised Japanese trial of triplet standard antiemetic therapies with or without 5 mg olanzapine to prevent chemotherapy-induced nausea and vomiting for patients with breast cancer treated with an anthracycline/cyclophosphamide regimen (JTOP-B). BMJ open, 12:e058755, 2022
8. Ogiwara T, Kawazoe H, Egami S, Hashimoto H, Saito Y, Sakiyama N, Ohe Y, Yamaguchi M, Furukawa T, Hara A, Hiraga Y, Jibiki A, Yokoyama Y, Suzuki S, Nakamura T. Prognostic Value of Baseline Medications Plus Neutrophil-to-Lymphocyte Ratio in the Effectiveness of Nivolumab and Pembrolizumab in Patients With Advanced Non-Small-Cell Lung Cancer: A Retrospective Study. Frontiers in oncology, 11:770268, 2021
9. Egami S, Kawazoe H, Hashimoto H, Uozumi R, Arami T, Sakiyama N, Ohe Y, Nakada H, Aomori T, Ikemura S, Fukunaga K, Yamaguchi M, Nakamura T. Absolute Lymphocyte Count Predicts Immune-Related Adverse Events in Patients With Non-Small-Cell Lung Cancer Treated With Nivolumab Monotherapy: A Multicenter Retrospective Study. Frontiers in oncology, 11:618570, 2021
10. Yanai Y, Makihara RA, Matsunaga N, Shimizu R, Tominaga S, Hoshino S, Nishibuchi Y, Maruki Y, Ohba A, Shimizu K, Okusaka T. A feasibility study of a peer discussion group intervention for patients with pancreatobiliary cancer and their caregivers. Palliative & supportive care, 1-8, 2021
11. Iwaki M, Kessoku T, Kanamori T, Abe K, Takeno N, Kawahara R, Fujimoto T, Igarashi T, Kumakura Y, Suzuki N, Kamiya K, Suzuki N, Tagami K, Saeki T, Mawatari H, Sakurai H, Higashibata T, Hirohashi T, Nakajima A, Ichikawa Y, Ishiki H. Tapentadol Safety and Patient Characteristics Associated with Treatment Discontinuation in Cancer Therapy: A Retrospective Multicentre Study in Japan. Pain and therapy, 10:1635-1648, 2021
12. Arami T, Kawazoe H, Uozumi R, Hashimoto H, Egami S, Sakiyama N, Ohe Y, Nakada H, Aomori T, Ikemura S, Yasuda H, Kawada I, Fukunaga K, Soejima K, Yamaguchi M, Nakamura T. Effect of renin-angiotensin system inhibitors on pemetrexed plus platinum-induced hematological toxicities: a multicenter retrospective study using three propensity score analyses. Die Pharmazie, 76:266-271, 2021
13. Shimanuki Y, Hashimoto H, Kawazoe H, Uozumi R, Udagawa R, Watabe D, Nakamura T, Yamaguchi M, Terakado H. Preventive effects of self-administered cryotherapy on paclitaxel-induced peripheral neuropathy in patients with early-stage breast cancer: a propensity score analysis. Die Pharmazie, 76:261-265, 2021
14. Abe K, Higurashi T, Takahashi M, Maeda-Minami A, Kawano Y, Miyazaki S, Mano Y. Concomitant Use of High-dose Methotrexate and Glycyrrhizin Affects Pharmacokinetics of Methotrexate, Resulting in Hepatic Toxicity. In vivo (Athens, Greece), 35:2163-2169, 2021
