Annual Report 2021
Division of Cancer Biology
Hirofumi Arakawa, Yasuyuki Nakamura, Naoki Ikari, Yoko Sagami, Katsuko Honjo
Research activities
p53/Mieap-regulated mitochondrial quality control
Mitochondria-eating protein (Mieap, also designated as SPATA18) was originally identified as a p53-inducible protein; its mRNA expression is directly regulated by the tumor suppressor p53 in response to various cellular stresses, including DNA damage (PLoS ONE 6: e16054, 2011). Mieap expression is lost in nearly 50% of human cancer cell lines due to promoter methylation. Mieap-deficient LS174T colorectal cancer cells exhibit increased mitochondrial reactive oxygen species (ROS) generation and decreased mitochondrial ATP synthesis. The mitochondrial ROS in the Mieap-deficient colorectal cancer and gastric cancer cells enhance migration and invasion activities of the cancer cells under hypoxic conditions (Oncogenesis 5: e181, 2016; Scientific Reports 9: 2822, 2019). Mieap-deficient ApcMin/+ mice exhibit the promotion of intestinal tumor initiation and malignant transformation (Scientific Reports 5: 12472, 2015). The tumors in Mieap-deficient ApcMin/+ mice have revealed abnormal morphology of the mitochondria, such as enlargement of the size, round shape, and decrease in cristae structures. Recently, Mieap expression was reported to be defective in thyroid oncocytic cell tumors, which exhibit accumulation of abnormal mitochondria in tumor cells (Cancer Sci. 111: 2814-2823, 2020). Mieap-regulated mitochondrial quality control is inactivated in the tumor tissues of nearly 70% of colorectal cancer, 25% of breast cancer, 70% of gastric cancer, and 83% of esophageal cancer patients (Oncogenesis 5: e181, 2016; Cancer Sci. 109: 3910-3920, 2018; Biochem Biophys Res Commun. 529: 582-589, 2020). Mieap is associated with better survival in breast cancer patients (Am J Cancer Res. 11: 6060-6073, 2021). These observations suggest that Mieap plays a critical role in tumor suppression via its mitochondrial quality control function. However, the mechanism underlying the regulation of mitochondrial quality control by Mieap remains unclear.
Liquid droplets function as membrane-less organelles in cells
Liquid droplets (LDs) in cells are protein condensates, which are composed of proteins, nucleic acids, and other macromolecular components. LDs are formed by proteins with an intrinsically disordered region (or regions) (IDR(s)) via liquid-liquid phase separation (LLPS) (Figure 1). Importantly, LDs function as membrane-less organelles that compartmentalize and facilitate cellular biological reactions. LDs are not surrounded by a lipid bilayer structure, which enables dynamic exchange of reactants and products with their surroundings, facilitating biological reactions in cells. Furthermore, theoretically, LDs are able to appear and disappear anywhere within a cell in response to cellular stress and/or subcellular circumstances, exhibiting spatiotemporally dynamic properties. On the basis of these features, the concept of membrane-less organelles is fundamentally different from the long-standing concept of membrane-bound organelles, such as the nucleus, lysosomes, mitochondria, and endoplasmic reticulum, which stably exist and strictly segregate biomolecules and biological reactions by means of a lipid bilayer structure. It is suggested that a number of membrane-less organelles may exist within a cell, which could regulate various cellular biological reactions and activities, including transcription, signal transduction, immunity, centrosome activity, and mitosis. This list is now rapidly growing.
Figure 1. Liquid droplets are induced by liquid-liquid phase
separation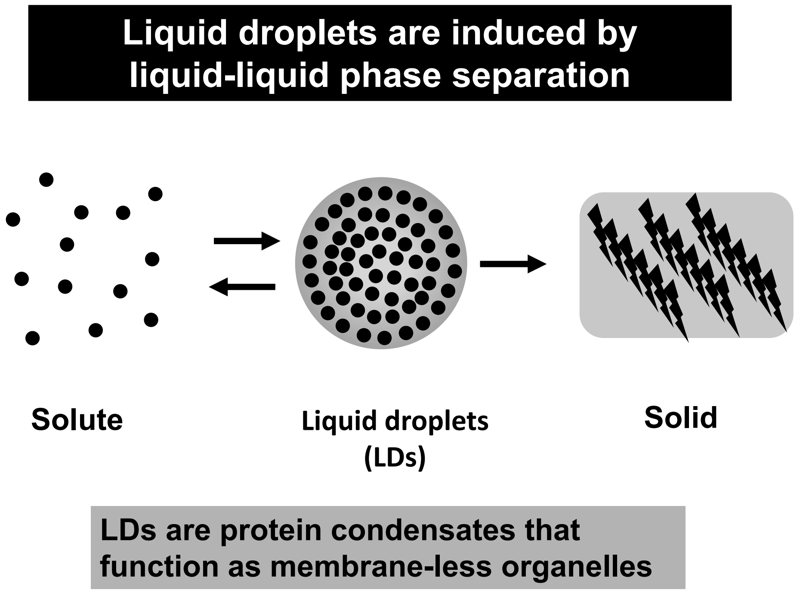

Cardiolipin (CL) is a unique and important phospholipid that is specific to mitochondria
CL is a phospholipid dimer that has two phosphate residues and four fatty acyl chains. Because of this unique structure, it forms a cone shape, which plays a critical role in forming the curvature of the lipid membrane, contributing to the maintenance of the mitochondrial cristae structures. CL is the only phospholipid that is specific to the mitochondria and is mainly located at the inner mitochondrial membrane and contact sites. CL interacts with many mitochondrial membrane proteins, including the electron transport chain complexes involved in oxidative phosphorylation and the ADP/ATP carrier; the interaction with CL promotes the activity of these proteins. CL stabilizes the structural assembly and activity of the respiratory super-complexes at the inner mitochondrial membrane. CL interacts with cytochrome c at the outer surface of the inner mitochondrial membrane, which plays a pivotal role in the life and death of cells by maintaining stable respiratory ATP production and inducing apoptosis, respectively. Therefore, altered CL metabolism and/or deterioration of CL quality causes various mitochondrial dysfunctions and diseases via abnormal cristae structures, decreased ATP synthesis activity, and increased ROS generation (Figure 2).
Figure 2. Role of cardiolipin in mitochondrial proteins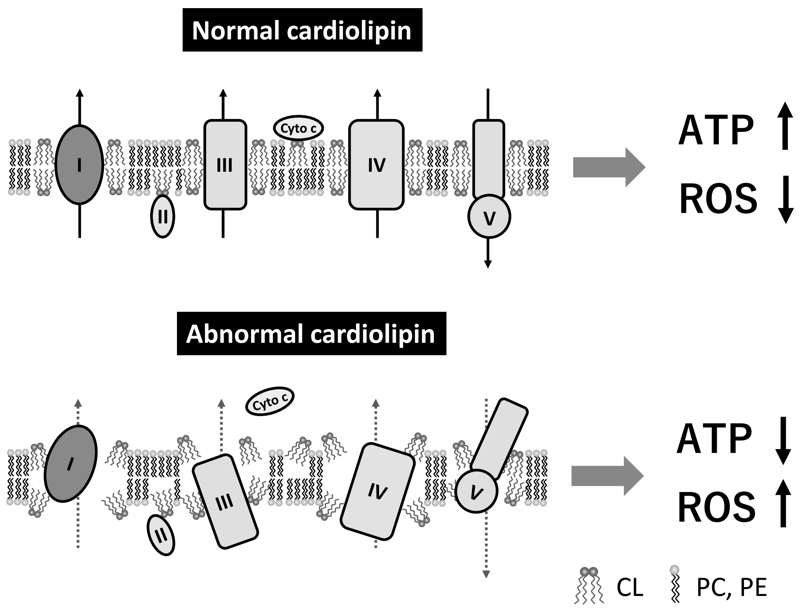

Mieap forms membrane-less organelles to compartmentalize and facilitate cardiolipin metabolism
This year, we reported that Mieap is an IDR-containing protein that drives the formation of liquid droplets in the mitochondria (bioRxiv doi: 10.1101/2020.10.26.354365). Mieap liquid droplets (MLDs) specifically phase separate the mitochondrial phospholipid cardiolipin. Lipidomic analysis of cardiolipin suggested that Mieap promotes enzymatic reactions involved in cardiolipin metabolism, including biosynthesis and remodeling. Accordingly, four cardiolipin biosynthesis enzymes, TAMM41, PGS1, PTPMT1, and CRLS1, and two remodeling enzymes, PLA2G6 and TAZ, are phase separated by MLDs. Mieap-deficient mice exhibited altered cristae structures in the liver and kidney mitochondria and a trend of obesity. These results suggest that Mieap drives the formation of membrane-less organelles to compartmentalize and promotes cardiolipin metabolism at the inner mitochondrial membrane, thus playing a possible role in mitochondrial quality control (Figure 3).
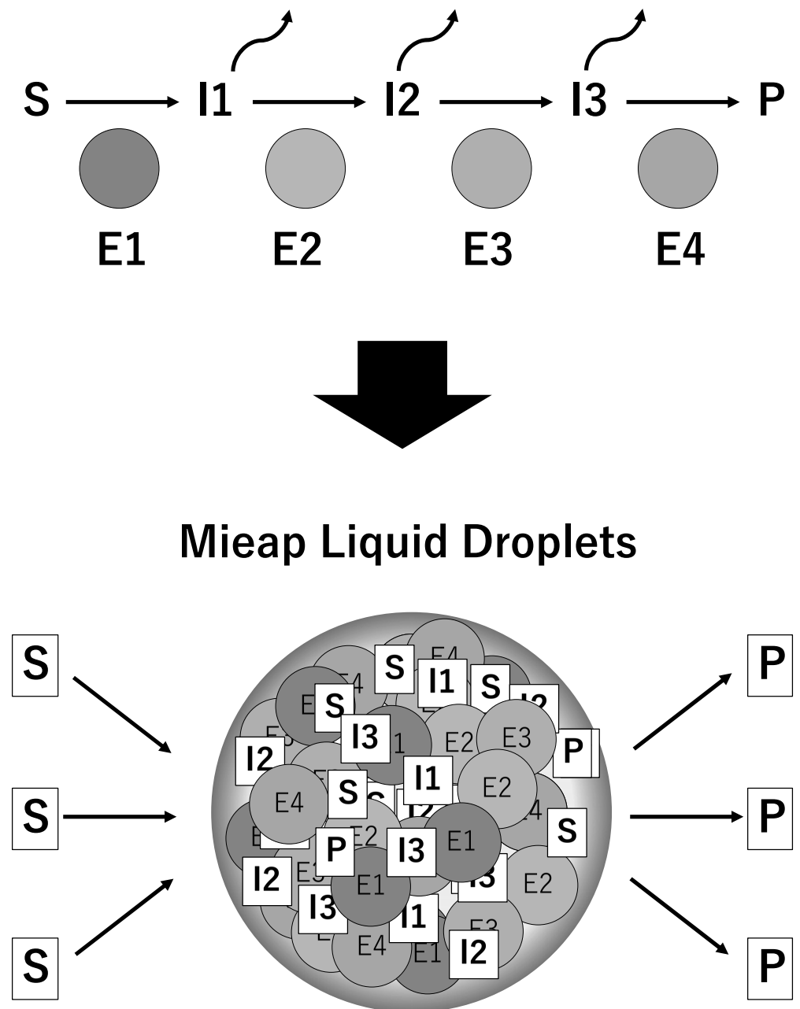
Future Prospects
So far, autophagy and proteostasis have been identified as two major mechanisms in mitochondrial quality control. In addition to these, we suggest that the Mieap-regulated pathway is a third mechanism for mitochondrial quality control, in which Mieap maintains the integrity of mitochondria by regulating CL metabolism. Mieap maintains mitochondrial quality through homeostasis of the inner mitochondrial membrane by regulating CL metabolism, stabilizing oxidative phosphorylation, and suppressing mitochondrial ROS generation (Figure 4). Considering the role of Mieap in p53 function, we suggest that Mieap could act as a spatiotemporally dynamic regulator/modulator in CL metabolism to suppress tumor initiation and progression.
Figure 4.p53/Mieap suppresses tumors by regulating CL
metabolism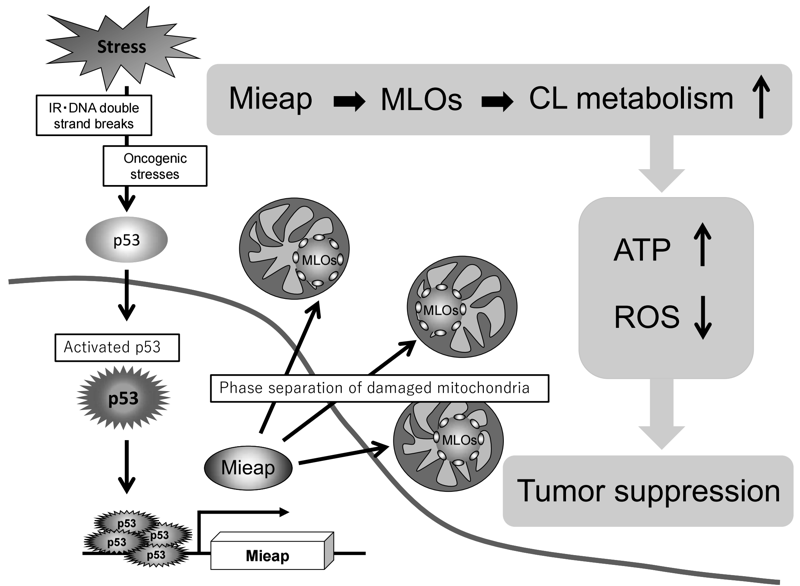

List of papers published in 2021
Journal
1. Futamura M, Tokumaru Y, Takabe K, Arakawa H, Asano Y, Mori R, Mase J, Nakakami A, Yoshida K. MIEAP, a p53-downstream gene, is associated with suppression of breast cancer cell proliferation and better survival. American journal of cancer research, 11:6060-6073, 2021
