Annual Report 2022
Section of Data Science Strategy
Takashi Kohno, Hirokazu Fukuda, Katsuya Tsuchihara, Genta Ohno, Haruka Nakada, Kaori Muto, Akiko Nagai, Yuuta Maruki, Misaki Ogawa
The Team and What We Do
Regarding comprehensive genomic profiling (CGP) tests that are performed for cancer patients under national health insurance in Japan, we promote the primary and secondary utilization of those genomic and clinical data collected at the Center for Cancer Genomics and Advanced Therapeutics (C-CAT) through various data-sharing systems (Figure 1). We perform the following tasks to contribute to the support and development of cancer genomic medicine (CGM):
1) Modification and operation of the “C-CAT Medical-Use Portal” to support medical treatment at the CGM hospitals.
2) Modification and operation of the “C-CAT Research-Use Portal” for academic research and development of pharmaceuticals, etc.
3) Construction of an additional cloud-based system, “C-CAT CALICO (CALculation & Investigation ClOud)”, which enables users to analyze raw sequence data to examine novel genetic alterations that are not included in the reports provided by the registered testing companies.
4) Secretariat management of the C-CAT Data Utilization Review Board to examine the suitability of applications for secondary utilization.
5) Public relations activities about CGM and CGP tests for patients and the general public.
Figure 1. Medical and Research Use of C-CAT “Real World Data”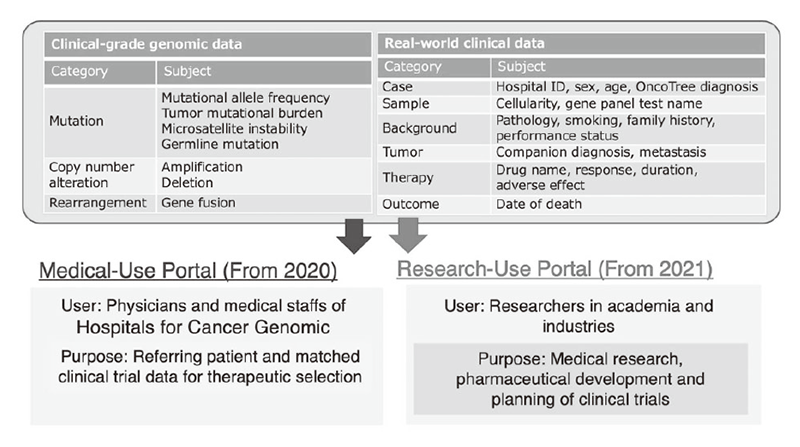

Figure 1. Medical and Research Use of C-CAT “Real World Data”. Both the genomic and clinical data can be accessed via two portal sites, the Medical-Use Portal for physicians and medical staff of the CGM hospitals and the Research-Use Portal for researchers in academia and industries.
Activities
- We started operating the official version of the “C-CAT Medical-Use Portal” in the previous year, and we continued to make appropriate system modifications to improve search capabilities and operability, and ensured stable operation.
- We began operation of the “C-CAT Research-Use Portal” in last October, and we conducted a user survey to improve the accuracy of the collected data and enhance the portal's functions. Based on the user responses, we improved usability through appropriate system modifications.
- In FY2022, the C-CAT Data Utilization Review Board were held four times, and 30 new applications from academic and industrial institutions in Japan (including Japanese branches of foreign pharmaceutical companies) were approved to utilize the C-CAT data. Additionally, two applications for research plan change were consulted and approved for continuous data utilization by the C-CAT Data Utilization Review Board.
- Based on the security requirements, we constructed an additional cloud-based system “C-CAT CALICO”, and prepared for operation.
- At the same time as expanding the website “CGM and CGP tests” for patients and the general public, we continued public relations activities through newsletters and Twitter, providing various information about CGP and the number of C-CAT registrants, etc.
Education
We are working to develop human resources in this field by giving lectures on CGM and the role of C-CAT at scientific conferences, as well as hosting tours from academic and medical institutes in Japan and overseas.
Future Prospects
We aim to further increase the number of users of the “C-CAT Research-Use Portal”. In preparation for the start of the “C-CAT CALICO” operation, final adjustments will be made regarding practical requirements such as usage fees and securing budget. In order to accommodate use from overseas, we will sort out legal issues related to the handling of sensitive personal information and establish both required systems and practical operations.
