Annual Report 2022
Department of Head and Neck Medical Oncology
Makoto Tahara, Susumu Okano, Tomohiro Enokida, Takao Fujisawa, Naohiro Takeshita, Nobukazu Tanaka, Yoh-ichiro Iwasa, Ryutaro Onaga, Yuta Hoshi
Introduction
The Department of Head and Neck Medical Oncology is engaged in the clinical management of patients with head and neck cancer (HNC), and research into anticancer drugs for the treatment of HNC.
Our missions are to: 1) provide the best evidence-based treatment; 2) promote the importance of supportive care in the treatment of patients with HNC; 3) facilitate the timely approval of new drugs by active participation in global clinical trials to eliminate the drug lag; 4) develop cutting-edge treatments; and 5) train experts in head and neck medical oncology.
The Team and What We Do
Our department consists of four physicians, two senior residents and three residents. We manage the treatment of patients with HNC who receive anticancer drugs. An estimated 60% of patients with HNC require a multidisciplinary approach, including surgery, radiotherapy, and chemotherapy. Given the increasing complexity of HNC management, we decide on the recommended treatment for patients who are referred to our institution at the weekly tumor board attended by a multidisciplinary team.
A total of 299 patients were referred to our department from April 2022 to March 2023 (Tables 1 and 2). The outpatient service of our department is available from Monday to Friday. We carefully follow patients during and after treatment and provide palliative chemotherapy as an outpatient service.
Table 1. Number of patients by site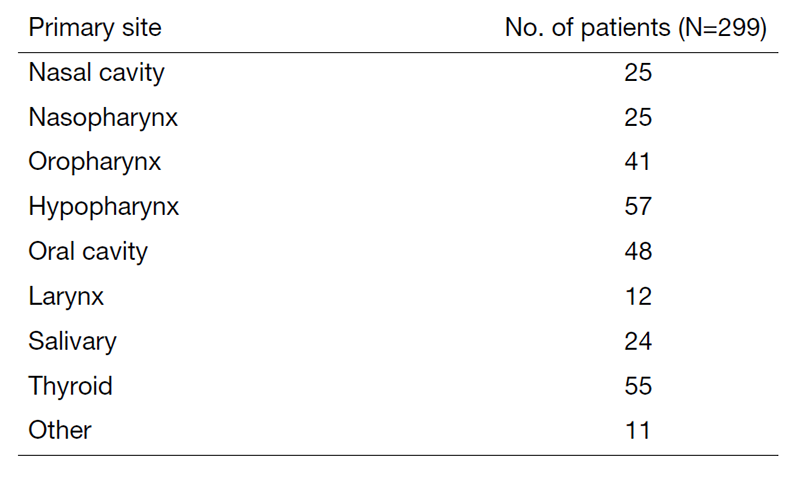

Table 2. Number of patients by procedure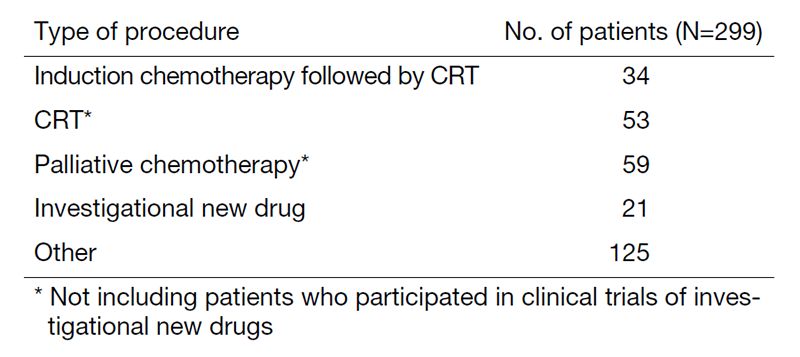

Research Activities
Our research activities have focused on three areas: the development of new treatments in clinical trials for HNC, biomarker analysis in HNC and retrospective analysis of HNC treatment.
We retrospectively assessed 29 patients with locoregionally advanced nasopharyngeal cancer (LA-NPC) who received the combination of paclitaxel, carboplatin, and cetuximab as induction chemotherapy (IC-PCE) at our institute from March 2017 to April 2021 (Takeshita N, Front Oncol, 2022). The response rate was 75.9% by IC-PCE and 100% at completion of CRT. The 3-year recurrence-free survival, locoregional failure-free survival, distant recurrence-free survival, and overall survival were 75.9%, 79.3%, 84.3%, and 96.3%, respectively. The incidence of adverse events in grade 3/4 was 34.5% during IC-PCE and 44.8% during CRT, respectively. In conclusion, IC-PCE is feasible and effective for LA-NPC and could be one of the treatment options for the disease.
We retrospectively reviewed the clinical records of five patients with recurrent or metastatic head and neck cancer (R/M HNC) who received biweekly cetuximab (Cmab) in our institute from January 2016 to September 2021 and compared the safety profile between two phases of weekly 250 mg/m2 and biweekly 500 mg/m2 of Cmab in the identical patients (Tanaka N, Int J Clin Oncol 2022). All patients initially received Cmab in combination with chemotherapy. Median dose of biweekly Cmab was 4 (3–12). Two patients demonstrated worsened (Grade ≥ 2) toxicities during biweekly Cmab, including one with grade 2 dry skin and the other with grade 2 skin infection. None developed grade ≥ 3 adverse events or discontinued treatment due to Cmab-related adverse events. In conclusion, biweekly Cmab was well tolerated and did not demonstrate severe toxicities related to Cmab for R/M HNC. These results indicate that biweekly Cmab is applicable for R/M HNC.
We retrospectively reviewed patients with platinum-refractory R/M SCCHN treated with nivolumab from May 2017 to March 2020 in our institute (Wada A, Eur J Cancer 2023). Of 110 patients identified, 56 received PPI and 24 received Abx within 30 days before or after initiation of nivolumab. The univariate analysis demonstrates a significant association between the use of PPI and Abx with poor prognosis in all parameters (PFS, PFS2, PFS3, and OS). Median OS (HR; 95% CI, p-value) by these covariates were 13.6 vs. 23.8 months (1.70; 1.01-2.87, p=0.046) for PPI and 10.0 vs. 20.1 months (1.85; 1.00-3.41, p=0.048) for Abx, respectively. In conclusion, the use of PPI and Abx attenuated the efficacy of nivolumab in R/M SCCHN.
Clinical Trials
The following investigator-initiated clinical trials are ongoing; 1) a phase 2 study of combination with nivolumab plus lenvatinib for unresectable anaplastic thyroid cancer, 2) a phase 2 study of darolutamide for androgen receptor positive recurrent or metastatic salivary gland carcinoma.
To facilitate the timely approval of new drugs and eliminate the drug lag, we have also participated in the global phase trials including immune-checkpoint inhibitors.
Education
We educate not only medical staff in our institute but also outside of our institute by conducting the education program, Seminar of the Japan Society of Supportive Care for patients with HNC. Furthermore, our department is accepting trainees at all times.
Future Prospects
We hope that ongoing or planned clinical trials will change the standard of care for HNC and our biomarker analysis will lead to the development of new treatment strategies. Our education program will increase the number of medical oncologist who takes charge of HNC treatment, leading to improving patient’s quality of survival.
List of papers published in 2022
Journal
1. Udagawa H, Takahashi S, Hirao M, Tahara M, Iwasa S, Sato Y, Hamakawa T, Shitara K, Horinouchi H, Chin K, Masuda N, Suzuki T, Okumura S, Takase T, Nagai R, Yonemori K. Liposomal eribulin for advanced adenoid cystic carcinoma, gastric cancer, esophageal cancer, and small cell lung cancer. Cancer medicine, 12:1269-1278, 2023
2. Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Brana I, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Lin J, Gumuscu B, Swaby RF, Rischin D. Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. Journal of clinical oncology, 41:790-802, 2023
3. Haddad RI, Harrington K, Tahara M, Ferris RL, Gillison M, Fayette J, Daste A, Koralewski P, Zurawski B, Taberna M, Saba NF, Mak M, Kawecki A, Girotto G, Alvarez Avitia MA, Even C, Toledo JGR, Guminski A, Müller-Richter U, Kiyota N, Roberts M, Khan TA, Miller-Moslin K, Wei L, Argiris A. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. Journal of clinical oncology, 41:2166-2180, 2023
4. Ferris RL, Harrington K, Schoenfeld JD, Tahara M, Esdar C, Salmio S, Schroeder A, Bourhis J. Inhibiting the inhibitors: Development of the IAP inhibitor xevinapant for the treatment of locally advanced squamous cell carcinoma of the head and neck. Cancer treatment reviews, 113:102492, 2023
5. Wada A, Enokida T, Okano S, Sato M, Tanaka H, Ueda Y, Fujisawa T, Takeshita N, Tanaka N, Tahara M. Proton pump inhibitors and antibiotics adversely effect the efficacy of nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. European journal of cancer (Oxford, England : 1990), 184:30-38, 2023
6. Shibutani Y, Suzuki S, Sagara A, Enokida T, Okano S, Fujisawa T, Sato F, Yumoto T, Sano M, Kawasaki T, Tahara M. Impact of lenvatinib-induced proteinuria and renal dysfunction in patients with thyroid cancer. Frontiers in oncology, 13:1154771, 2023
7. Chatterjee S, Kiyota N, Vaish R, Sharma A, Tahara M, Noronha V, Prabhash K, D’Cruz A. Weekly versus 3-weekly cisplatin along with radiotherapy for locoregionally advanced non-nasopharyngeal head and neck cancers: Is the equipoise in literature addressed yet? Head & neck, 45:1594-1603, 2023
8. Harrington KJ, Ferris RL, Gillison M, Tahara M, Argiris A, Fayette J, Schenker M, Bratland Å, Walker JWT, Grell P, Even C, Chung CH, Redman R, Coutte A, Salas S, Grant C, de Azevedo S, Soulières D, Hansen AR, Wei L, Khan TA, Miller-Moslin K, Roberts M, Haddad R. Efficacy and Safety of Nivolumab Plus Ipilimumab vs Nivolumab Alone for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: The Phase 2 CheckMate 714 Randomized Clinical Trial. JAMA oncology, e230147, 2023
9. Kiyota N, Tahara M, Mizusawa J, Kodaira T, Fujii H, Yamazaki T, Mitani H, Iwae S, Fujimoto Y, Onozawa Y, Hanai N, Ogawa T, Hara H, Monden N, Shimura E, Minami S, Fujii T, Tanaka K, Homma A, Yoshimoto S, Oridate N, Omori K, Ueda T, Okami K, Ota I, Shiga K, Sugasawa M, Asakage T, Saito Y, Murono S, Nishimura Y, Nakamura K, Hayashi R. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. Journal of clinical oncology, 40:1980-1990, 2022
10. Kato T, Matsubara N, Shiota M, Eto M, Osawa T, Abe T, Shinohara N, Yasumizu Y, Tanaka N, Oya M, Nishimoto K, Hayashi T, Nakayama M, Kojima T, Namikawa K, Fujisawa T, Okano S, Hida E, Nakamura Y, Bando H, Yoshino T, Nonomura N. IMAGENE trial: multicenter, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy of niraparib with PD-1 inhibitor in solid cancer patients with homologous recombination repair genes mutation. BMC cancer, 22:1292, 2022
11. Doki Y, Ueno M, Hsu CH, Oh DY, Park K, Yamamoto N, Ioka T, Hara H, Hayama M, Nii M, Komuro K, Sugimoto M, Tahara M. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head-and-neck cancer. Cancer medicine, 11:2550-2560, 2022
12. Imamura Y, Kiyota N, Tahara M, Hanai N, Asakage T, Matsuura K, Ota I, Saito Y, Sano D, Kodaira T, Motegi A, Yasuda K, Takahashi S, Yokota T, Okano S, Tanaka K, Onoe T, Ariizumi Y, Homma A. Systemic therapy for salivary gland malignancy: current status and future perspectives. Japanese journal of clinical oncology, 52:293-302, 2022
13. Saito Y, Homma A, Kiyota N, Tahara M, Hanai N, Asakage T, Matsuura K, Ota I, Yokota T, Sano D, Kodaira T, Motegi A, Yasuda K, Takahashi S, Tanaka K, Onoe T, Okano S, Imamura Y, Ariizumi Y, Hayashi R. Human papillomavirus-related oropharyngeal carcinoma. Japanese journal of clinical oncology, 52:700-706, 2022
14. Haddad RI, Seiwert TY, Chow LQM, Gupta S, Weiss J, Gluck I, Eder JP, Burtness B, Tahara M, Keam B, Kang H, Muro K, Albright A, Mogg R, Ayers M, Huang L, Lunceford J, Cristescu R, Cheng J, Mehra R. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. Journal for immunotherapy of cancer, 10:e003026, 2022
15. Burtness B, Rischin D, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Brana I, Basté N, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Ge J, Swaby RF, Gumuscu B, Harrington K. Pembrolizumab Alone or With Chemotherapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma in KEYNOTE-048: Subgroup Analysis by Programmed Death Ligand-1 Combined Positive Score. Journal of clinical oncology, 40:2321-2332, 2022
16. Kotani D, Nakamura Y, Fujisawa T, Bando H, Sakamoto N, Johns AL, Park K, Casolino R, Yoshino T, Biankin AV. ICGC-ARGO precision medicine: targeted therapy according to longitudinal assessment of tumour heterogeneity in colorectal cancer. The Lancet. Oncology, 23:463-464, 2022
17. Kiyota N, Tahara M, Robinson B, Schlumberger M, Sherman SI, Leboulleux S, Lee EK, Suzuki T, Ren M, Fushimi K, Wirth LJ. Impact of baseline tumor burden on overall survival in patients with radioiodine-refractory differentiated thyroid cancer treated with lenvatinib in the SELECT global phase 3 trial. Cancer, 128:2281-2287, 2022
18. Rischin D, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Braña I, Neupane P, Bratland Å, Fuereder T, Hughes BGM, Mesía R, Ngamphaiboon N, Rordorf T, Ishak WZW, Hong RL, Mendoza RG, Jia L, Chirovsky D, Norquist J, Jin F, Burtness B. Pembrolizumab alone or with chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma: Health-related quality-of-life results from KEYNOTE-048. Oral oncology, 128:105815, 2022
19. Wirth LJ, Durante C, Topliss DJ, Winquist E, Robenshtok E, Iwasaki H, Luster M, Elisei R, Leboulleux S, Tahara M. Lenvatinib for the Treatment of Radioiodine-Refractory Differentiated Thyroid Cancer: Treatment Optimization for Maximum Clinical Benefit. The oncologist, 27:565-572, 2022
20. Le X, Baik C, Bauman J, Gilbert J, Brose MS, Grilley-Olson JE, Patil T, McDermott R, Raez LE, Johnson JM, Shen L, Tahara M, Ho AL, Norenberg R, Dima L, Brega N, Drilon A, Hong DS. Larotrectinib Treatment for Patients With TRK Fusion-Positive Salivary Gland Cancers. The oncologist, oyac080, 2022
21. Ueda Y, Okano S, Enokida T, Fujisawa T, Ito K, Sato M, Tanaka H, Wada A, Tahara M. Nivolumab for recurrent or metastatic head and neck cancer patients with non-squamous cell carcinoma and/or a primary subsite excluded from CheckMate141, a retrospective study. Oral oncology, 130:105932, 2022
22. Gillison ML, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Jayaprakash V, Wei L, Ferris RL. Long-term Outcomes with Nivolumab as First-line Treatment in Recurrent or Metastatic Head and Neck Cancer: Subgroup Analysis of CheckMate 141. The oncologist, 27:e194-e198, 2022
23. Kurozumi K, Fujii K, Washio K, Ishida J, Otani Y, Sudo T, Tahara M, Ichimura K, Ennishi D, Date I. Response to entrectinib in a malignant glioneuronal tumor with ARHGEF2-NTRK fusion. Neuro-oncology advances, 4:vdac094, 2022
24. Higashiyama T, Sugino K, Hara H, Ito KI, Nakashima N, Onoda N, Tori M, Katoh H, Kiyota N, Ota I, Suganuma N, Hibi Y, Nemoto T, Takahashi S, Yane K, Ioji T, Kojima S, Kaneda H, Sugitani I, Tahara M. Phase II study of the efficacy and safety of lenvatinib for anaplastic thyroid cancer (HOPE). European journal of cancer (Oxford, England : 1990), 173:210-218, 2022
25. Tanaka N, Enokida T, Fujisawa T, Okano S, Wada A, Sato M, Tanaka H, Takeshita N, Tahara M. Biweekly administration of cetuximab in Japanese patients with recurrent or metastatic head and neck cancer. International journal of clinical oncology, 27:1669-1674, 2022
26. Wirth LJ, Brose MS, Elisei R, Capdevila J, Hoff AO, Hu MI, Tahara M, Robinson B, Gao M, Xia M, Maeda P, Sherman E. LIBRETTO-531: a phase III study of selpercatinib in multikinase inhibitor-naïve RET-mutant medullary thyroid cancer. Future oncology (London, England), 18:3143-3150, 2022
27. Takeshita N, Enokida T, Okano S, Fujisawa T, Wada A, Sato M, Tanaka H, Tanaka N, Motegi A, Zenda S, Akimoto T, Tahara M. Induction chemotherapy with paclitaxel, carboplatin and cetuximab for locoregionally advanced nasopharyngeal carcinoma: A single-center, retrospective study. Frontiers in oncology, 12:951387, 2022
28. Müller-Jensen L, Zierold S, Versluis JM, Boehmerle W, Huehnchen P, Endres M, Mohr R, Compter A, Blank CU, Hagenacker T, Meier F, Reinhardt L, Gesierich A, Salzmann M, Hassel JC, Ugurel S, Zimmer L, Banks P, Spain L, Soon JA, Enokida T, Tahara M, Kähler KC, Seggewiss-Bernhardt R, Harvey C, Long GV, Schöberl F, von Baumgarten L, Hundsberger T, Schlaak M, French LE, Knauss S, Heinzerling LM. Characteristics of immune checkpoint inhibitor-induced encephalitis and comparison with HSV-1 and anti-LGI1 encephalitis: A retrospective multicentre cohort study. European journal of cancer (Oxford, England : 1990), 175:224-235, 2022
29. Sakai SA, Aoshima M, Sawada K, Horasawa S, Yoshikawa A, Fujisawa T, Kadowaki S, Denda T, Matsuhashi N, Yasui H, Goto M, Yamazaki K, Komatsu Y, Nakanishi R, Nakamura Y, Bando H, Hamaya Y, Kageyama SI, Yoshino T, Tsuchihara K, Yamashita R. Fecal microbiota in patients with a stoma decreases anaerobic bacteria and alters taxonomic and functional diversities. Frontiers in cellular and infection microbiology, 12:925444, 2022
30. Mizuno M, Chiba I, Mukohara T, Kondo M, Maruo K, Ohigashi T, Naruo M, Asano Y, Onishi T, Tanabe H, Muta R, Mishima S, Okano S, Yuda M, Hosono A, Ueda Y, Bando H, Itagaki H, Ferrans CE, Akimoto T. Effectiveness of an online support program to help female cancer patients manage their health and illness: Protocol for a randomized controlled trial. Contemporary clinical trials communications, 30:101035, 2022
31. Müller-Jensen L, Zierold S, Versluis JM, Boehmerle W, Huehnchen P, Endres M, Mohr R, Compter A, Blank CU, Hagenacker T, Meier F, Reinhardt L, Gesierich A, Salzmann M, Hassel JC, Ugurel S, Zimmer L, Banks P, Spain L, Soon JA, Enokida T, Tahara M, Kähler KC, Seggewiss-Bernhardt R, Harvey C, Long GV, Schöberl F, von Baumgarten L, Hundsberger T, Schlaak M, French LE, Knauss S, Heinzerling LM. Dataset of a retrospective multicenter cohort study on characteristics of immune checkpoint inhibitor-induced encephalitis and comparison with HSV-1 and anti-LGI1 encephalitis. Data in brief, 45:108649, 2022
