Annual Report 2022
Department of Medical Oncology
Toru Mukohara, Ako Hosono, Yoichi Naito, Nobuaki Matsubara, Kenichi Harano, Chihiro Kondoh, Chikako Funasaka, Hiromichi Nakajima, Hirofumi Mukai, Takeriro Nakao, Shota Kusuhara, Misao Fukuda, Nobuyuki Takahashi, Akira Hirota, Hikari Kiyohara, Mao Uematsu
Introduction
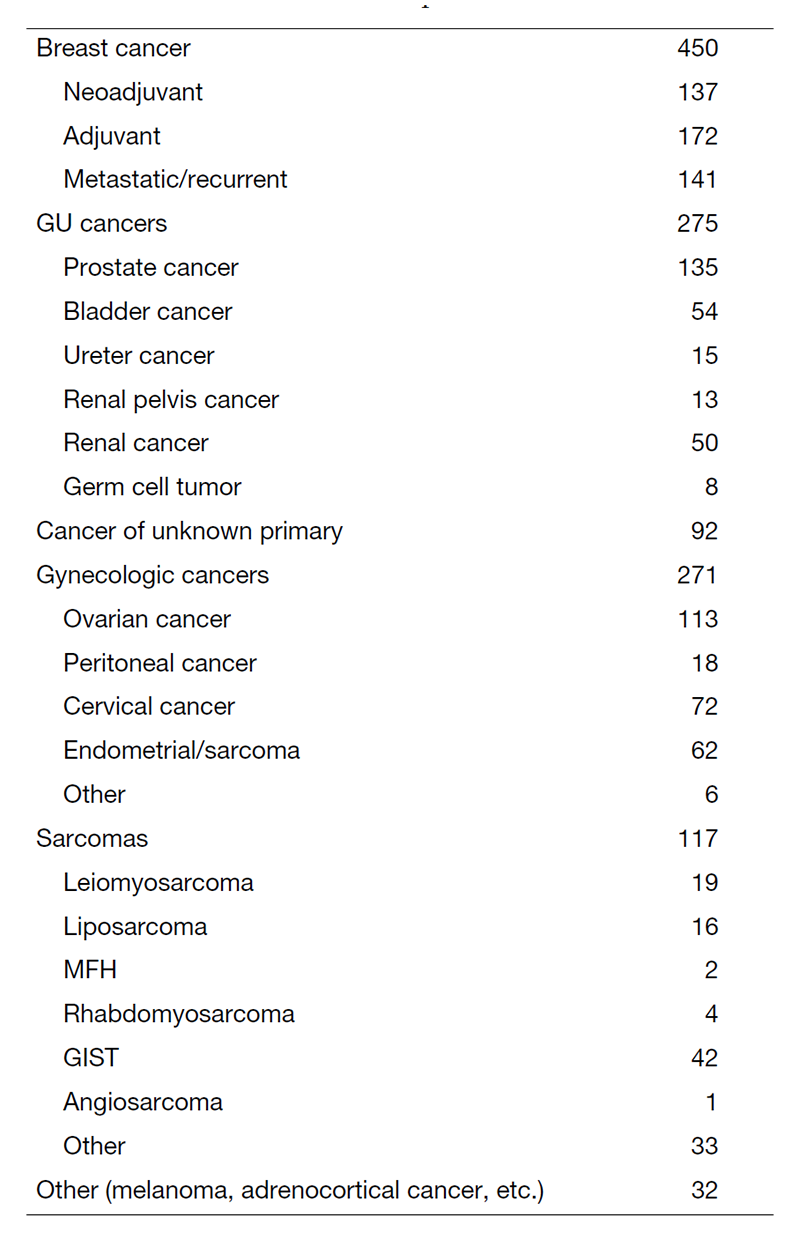
Our department is the only department in the hospital that provides care for cross-organ cancers, mainly breast cancer, urologic cancer, gynecologic cancer, and bone and soft tissue sarcoma. We also provide appropriate treatment for malignancies that are difficult to treat at other facilities, such as cancers of unknown primary, multiple, and rare cancers, utilizing our abundant experience as an oncologist. We also play a central role in conferences that include surgical departments and radiation therapy departments, such as the "Urologic Tumor Conference," "Bone and Soft Tissue Sarcoma Conference," "Gynecologic Tumor Conference," and "Breast Tumor Conference," and implement multidisciplinary treatment.
The Team and What We Do
In terms of medical care, the number of new patients was comparable to the record high in FY2021, and the average number of patients treated per day was the highest ever recorded. In addition, we were deeply involved in the operation of the cancer gene profiling test under the insurance reimbursement system as in the previous fiscal year. Dr. Mukohara, our department's chief, served as the head of the expert panel. In total, 263 (43%) of the 607 cases discussed by the expert panel in FY2022 were handled by our department. In addition, 161 of the cases were from collaborating hospitals, contributing to the implementation of genomic medicine not only in our hospital but also in other regions.
In terms of research, we are conducting both retrospective research and prospective clinical trials, new drug development trials, and translational research (TR) associated with these trials. The randomized, double-blind, investigator-initiated clinical trial of limb cooling therapy for prevention of peripheral neuropathy in breast cancer patients receiving paclitaxel therapy, which began enrollment last fiscal year, is progressing smoothly. Enrollment in an investigator-initiated trial evaluating olaparib plus pembrolizumab as preoperative chemotherapy for HRD-positive advanced ovarian cancer is also progressing well. TR applying single-cell RNA sequencing associated with the clinical trial is being developed in collaboration with the Genome TR Laboratory. In addition, registration for the investigator-initiated clinical trial of niraparib + pimitespib began this fiscal year. In addition, registration of joint research with a company on circulating tumor cells (CTCs) has been started.
Research Activities
We actively enrolled cases in company-initiated clinical trials, presented at international conferences, and had opportunities to be the first author and co-author of English-language journals. We have obtained public research funds such as Grant-in-Aid for Scientific Research "Development of a Predictive Model for Anti-Tumor Drug Susceptibility Using Patient-Derived Xenografts and 3D Primary Culture" (PI: Mukohara) and Grant-in-Aid for Scientific Research "Study on a New Subclass to Assess Immunocompetence in Triple Negative Breast Cancer and Its Clinical Usefulness" (PI Furukawa), which are underway.
Clinical Trials
IITs we are leading
- Phase I Trial of Niraparib and Pimitespib in Patients with Solid Tumors (PI, Naito Y) : jRCT2031220179
- A randomized Control trial to Evaluate mitigation of CIPN by limb-cooling apparatus in breast cancer patients who undergo weekly paclitaxel (CECILIA) (PI, Mukohara T): jRCT2032210115
- Phase I clinical study to evaluate the safety and tolerability of intraperitoneal administration of anti-GPC3-CAR expressing iPS cell-derived ILC/NK cells in patients with inoperable advanced recurrent ovarian clear cell carcinoma expressing GPC3 and with peritoneal dissemination (PI, Harano K): jRCT2033200431
- Double-blind, placebo-controlled, randomized, phase III trial of paclitaxel plus carboplatin plus atezolizumab in patients with advanced/recurrent uterine cancer (PI, Harano K): jRCT2031190013
- Olaparib Monotherapy and Olaparib + Pembrolizumab Combination Therapy for Ovarian Cancer (OLAPem) (PI, Harano K): NCT04417192
Education
The goal of our training and education is to develop true medical oncologists. Residents who graduate from our department are expected to be able to provide the standard of care for all types of cancer and to work collaboratively with other medical staff in the daily practice of medicine. They must are also conduct research and write papers to address clinical questions that they identify during their residency. In FY2022, two papers were published in English with the residents as first authors under the supervision of our staff. In addition, four residents were given the opportunity to make presentations at the Japanese Society of Clinical Oncology and other conferences.
Future Prospects
In the clinic, we will provide medical care with high patient satisfaction through multidisciplinary team medicine. In education, we will promote the training of medical oncologists who can treat patients across organ systems and scientific knowledge. In research, we will continue to lead development trials of new drugs from Phase I to Phase III. In addition, we will conduct preclinical research with the Genome TR laboratory on predicting drug efficacy and overcoming drug resistance, and aim to conduct investigator-initiated clinical trials based on the findings obtained. We will also continue to promote joint research with companies and other academic institutions.
List of papers published in 2022
Journal
1. Doi T, Shitara K, Kojima T, Kuboki Y, Matsubara N, Bando H, Yoh K, Naito Y, Hirai H, Kurokawa Y, Kato T, Morizane C. Phase I study of the irreversible fibroblast growth factor receptor 1-4 inhibitor futibatinib in Japanese patients with advanced solid tumors. Cancer science, 114:574-585, 2023
2. Shimomura A, Yoshida M, Kubo T, Yamashita S, Noguchi E, Nagayama A, Hanamura T, Okazaki M, Mukohara T, Tsuruga A, Tanaka K, Kawamura Y, Higuchi T, Takahashi Y, Kurozumi S, Hayashida T, Ichikawa H, Ushijima T, Suto A. Clinicopathological features, genetic alterations, and BRCA1 promoter methylation in Japanese male patients with breast cancer. Breast cancer research and treatment, 197:593-602, 2023
3. Tamura K, Mukohara T, Yonemori K, Kawabata Y, Nicolas X, Tanaka T, Iwata H. Phase 1 study of oral selective estrogen receptor degrader (SERD) amcenestrant (SAR439859), in Japanese women with ER-positive and HER2-negative advanced breast cancer (AMEERA-2). Breast cancer (Tokyo, Japan), 30:506-517, 2023
4. Kunisada T, Nakata E, Fujiwara T, Hosono A, Takihira S, Kondo H, Ozaki T. Soft-tissue sarcoma in adolescents and young adults. International journal of clinical oncology, 28:1-11, 2023
5. Matsubara N, Yonese J, Kojima T, Azuma H, Matsumoto H, Powles T, Rosenberg JE, Petrylak DP, Matsangou M, Wu C, Campbell M, Yamashiro M. Japanese subgroup analysis of EV-301: An open-label, randomized phase 3 study to evaluate enfortumab vedotin versus chemotherapy in subjects with previously treated locally advanced or metastatic urothelial carcinoma. Cancer medicine, 12:2761-2771, 2023
6. Kuroe T, Watanabe R, Morisue R, Miyazaki S, Kojima M, Murata SC, Nakai T, Taki T, Sakashita S, Sakamoto N, Matsubara N, Masuda H, Ushiku T, Ishii G. Dirty necrosis in renal cell carcinoma is associated with NETosis and systemic inflammation. Cancer medicine, 12:4557-4567, 2023
7. Tsuzuki T, Ohe C, Osawa T, Yasuda Y, Tanaka T, Anai S, Kimura G, Yamana K, Hatakeyama S, Yoshimoto T, Nakagawa Y, Fukuyama T, Matsubara N, Uemura H. Prognostic value of immune phenotype and PD-L1 status in recurrent or metastatic renal cell carcinoma: an exploratory analysis of the ARCHERY study. Pathology, 55:31-39, 2023
8. Matsubara N, de Bono J, Olmos D, Procopio G, Kawakami S, Ürün Y, van Alphen R, Flechon A, Carducci MA, Choi YD, Hotte SJ, Korbenfeld E, Kramer G, Agarwal N, Chi KN, Dearden S, Gresty C, Kang J, Poehlein C, Harrington EA, Hussain M. Olaparib Efficacy in Patients with Metastatic Castration-resistant Prostate Cancer and BRCA1, BRCA2, or ATM Alterations Identified by Testing Circulating Tumor DNA. Clinical cancer research, 29:92-99, 2023
9. Funasaka C, Naito Y, Kusuhara S, Nakao T, Nakajima H, Kawamoto M, Baba K, Mamishin K, Kondoh C, Harano K, Matsubara N, Hosono A, Sasaki T, Kawasaki T, Mukohara T. Clinical features of CDK4/6 inhibitor-related interstitial lung disease in patients with breast cancer: a case series study. Japanese journal of clinical oncology, 53:105-114, 2023
10. Koganemaru S, Kawai T, Fuchigami H, Maeda N, Koyama K, Kuboki Y, Mukohara T, Doi T, Yasunaga M. Quantitative analysis of drug distribution in heterogeneous tissues using dual-stacking capillary electrophoresis-mass spectrometry. British journal of pharmacology, 180:762-774, 2023
11. Tamada S, Nozawa M, Ohba K, Mizuno R, Takamoto A, Ohe C, Yoshimoto T, Nakagawa Y, Fukuyama T, Matsubara N, Kimura G, Tomita Y, Nonomura N, Eto M. Prognostic value of PD-L1 expression in recurrent renal cell carcinoma after nephrectomy: a secondary analysis of the ARCHERY study. International journal of clinical oncology, 28:289-298, 2023
12. Matsubara N, de Bono J, Sweeney C, Chi KN, Olmos D, Sandhu S, Massard C, Garcia J, Chen G, Harris A, Schenkel F, Sane R, Hinton H, Bracarda S, Sternberg CN. Safety Profile of Ipatasertib Plus Abiraterone vs Placebo Plus Abiraterone in Metastatic Castration-resistant Prostate Cancer. Clinical genitourinary cancer, 21:230-237.e1, 2023
13. Naito Y, Nishida T, Doi T. Current status of and future prospects for the treatment of unresectable or metastatic gastrointestinal stromal tumours. Gastric cancer, 26:339-351, 2023
14. Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA oncology, 8:1447-1455, 2022
15. Ohashi Y, Ikeda M, Kunitoh H, Sasako M, Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, Ibusuki K, Takita A, Sakon M. One-year incidence of venous thromboembolism, bleeding, and death in patients with solid tumors newly initiating cancer treatment: Results from the Cancer-VTE Registry. Thrombosis research, 213:203-213, 2022
16. Kato T, Matsubara N, Shiota M, Eto M, Osawa T, Abe T, Shinohara N, Yasumizu Y, Tanaka N, Oya M, Nishimoto K, Hayashi T, Nakayama M, Kojima T, Namikawa K, Fujisawa T, Okano S, Hida E, Nakamura Y, Bando H, Yoshino T, Nonomura N. IMAGENE trial: multicenter, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy of niraparib with PD-1 inhibitor in solid cancer patients with homologous recombination repair genes mutation. BMC cancer, 22:1292, 2022
17. Imai M, Nakamura Y, Sunami K, Kage H, Komine K, Koyama T, Amano T, Ennishi D, Kanai M, Kenmotsu H, Maeda T, Morita S, Sakai D, Bando H, Makiyama A, Suzuki T, Hirata M, Kohsaka S, Tsuchihara K, Naito Y, Yoshino T. Expert panel consensus recommendations on the use of circulating tumor DNA assays for patients with advanced solid tumors. Cancer science, 113:3646-3656, 2022
18. Sunami K, Naito Y, Komine K, Amano T, Ennishi D, Imai M, Kage H, Kanai M, Kenmotsu H, Koyama T, Maeda T, Morita S, Sakai D, Kohsaka S, Tsuchihara K, Saigusa Y, Yoshino T. Chronological improvement in precision oncology implementation in Japan. Cancer science, 113:3995-4000, 2022
19. Naito Y, Sunami K, Kage H, Komine K, Amano T, Imai M, Koyama T, Ennishi D, Kanai M, Kenmotsu H, Maeda T, Morita S, Sakai D, Watanabe K, Shirota H, Kinoshita I, Yoshioka M, Mamesaya N, Ito M, Kohsaka S, Saigusa Y, Yamamoto K, Hirata M, Tsuchihara K, Yoshino T. Concordance Between Recommendations From Multidisciplinary Molecular Tumor Boards and Central Consensus for Cancer Treatment in Japan. JAMA network open, 5:e2245081, 2022
20. Nishio S, Yonemori K, Usami T, Minobe S, Yunokawa M, Iwata T, Okamoto A, Aoki Y, Itamochi H, Takekuma M, Harano K, Yamamoto K, Maruko T, Ugai H, Tekin C, Colombo N, Fujiwara K, Hasegawa K, Ushijima K. Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: Results from KEYNOTE-826. Cancer science, 113:3877-3887, 2022
21. Masuda N, Ono M, Mukohara T, Yasojima H, Shimoi T, Kobayashi K, Harano K, Mizutani M, Tanioka M, Takahashi S, Kogawa T, Suzuki T, Okumura S, Takase T, Nagai R, Semba T, Zhao ZM, Ren M, Yonemori K. Phase 1 study of the liposomal formulation of eribulin (E7389-LF): Results from the breast cancer expansion cohort. European journal of cancer (Oxford, England : 1990), 168:108-118, 2022
22. Kotani H, Masuda N, Yamashita T, Naito Y, Taira T, Inoue K, Takahashi M, Yonemori K, Toyoizumi S, Mori Y, Nagasawa T, Hori N, Iwata H. Efficacy and safety of talazoparib in Japanese patients with germline BRCA-mutated locally advanced or metastatic breast cancer: results of the phase 1 dose-expansion study. Breast cancer (Tokyo, Japan), 29:1088-1098, 2022
23. Mizuno M, Chiba I, Mukohara T, Kondo M, Maruo K, Ohigashi T, Naruo M, Asano Y, Onishi T, Tanabe H, Muta R, Mishima S, Okano S, Yuda M, Hosono A, Ueda Y, Bando H, Itagaki H, Ferrans CE, Akimoto T. Effectiveness of an online support program to help female cancer patients manage their health and illness: Protocol for a randomized controlled trial. Contemporary clinical trials communications, 30:101035, 2022
24. Rini BI, Atkins MB, Plimack ER, Soulières D, McDermott RS, Bedke J, Tartas S, Alekseev B, Melichar B, Shparyk Y, Kondoh C, Langiewicz P, Wood LA, Hammers H, Silber CG, Haber B, Jensen E, Chen M, Powles T. Characterization and Management of Treatment-emergent Hepatic Toxicity in Patients with Advanced Renal Cell Carcinoma Receiving First-line Pembrolizumab plus Axitinib. Results from the KEYNOTE-426 Trial. European urology oncology, 5:225-234, 2022
25. Kawahara T, Taira N, Shiroiwa T, Hagiwara Y, Fukuda T, Uemura Y, Mukai H. Minimal important differences of EORTC QLQ-C30 for metastatic breast cancer patients: Results from a randomized clinical trial. Quality of life research, 31:1829-1836, 2022
26. de Wit R, Powles T, Castellano D, Necchi A, Lee JL, van der Heijden MS, Matsubara N, Bamias A, Fléchon A, Sternberg CN, Drakaki A, Yu EY, Zimmermann AH, Long A, Walgren RA, Gao L, Bell-McGuinn KM, Petrylak DP. Exposure-response relationship of ramucirumab in RANGE, a randomized phase III trial in advanced urothelial carcinoma refractory to platinum therapy. British journal of clinical pharmacology, 88:3182-3192, 2022
27. Hussain M, Corcoran C, Sibilla C, Fizazi K, Saad F, Shore N, Sandhu S, Mateo J, Olmos D, Mehra N, Kolinsky MP, Roubaud G, Özgüroğlu M, Matsubara N, Gedye C, Choi YD, Padua C, Kohlmann A, Huisden R, Elvin JA, Kang J, Adelman CA, Allen A, Poehlein C, de Bono J. Tumor Genomic Testing for >4,000 Men with Metastatic Castration-resistant Prostate Cancer in the Phase III Trial PROfound (Olaparib). Clinical cancer research, 28:1518-1530, 2022
28. Eguchi Y, Nakai T, Kojima M, Wakabayashi M, Sakamoto N, Sakashita S, Miyazaki S, Taki T, Watanabe R, Watanuki R, Yamauchi C, Iwatani T, Mukohara T, Onishi T, Ishii G. Pathologic method for extracting good prognosis group in triple-negative breast cancer after neoadjuvant chemotherapy. Cancer science, 113:1507-1518, 2022
29. van der Heijden MS, Powles T, Petrylak D, de Wit R, Necchi A, Sternberg CN, Matsubara N, Nishiyama H, Castellano D, Hussain SA, Bamias A, Gakis G, Lee JL, Tagawa ST, Vaishampayan U, Aragon-Ching JB, Eigl BJ, Hozak RR, Rasmussen ER, Xia MS, Rhodes R, Wijayawardana S, Bell-McGuinn KM, Aggarwal A, Drakaki A. Predictive biomarkers for survival benefit with ramucirumab in urothelial cancer in the RANGE trial. Nature communications, 13:1878, 2022
30. Kazama H, Kawaguchi O, Seto T, Suzuki K, Matsuyama H, Matsubara N, Tajima Y, Fukao T. Comprehensive analysis of the associations between clinical factors and outcomes by machine learning, using post marketing surveillance data of cabazitaxel in patients with castration-resistant prostate cancer. BMC cancer, 22:470, 2022
31. Thomas A, Desai P, Takahashi N. Translational research: A patient-centered approach to bridge the valley of death. Cancer cell, 40:565-568, 2022
32. Yasui H, Takeno A, Hara H, Imamura H, Akamatsu H, Fujitani K, Nakane M, Kondoh CN, Yukisawa S, Nasu J, Miyata Y, Makiyama A, Ishida H, Yoshida N, Matsumura E, Ishigami M, Sugihara M, Ochiai A, Doi T. Prospective analysis of the expression status of FGFR2 and HER2 in colorectal and gastric cancer populations: DS-Screen Study. International journal of colorectal disease, 37:1393-1402, 2022
33. Roubaud G, Özgüroğlu M, Penel N, Matsubara N, Mehra N, Kolinsky MP, Procopio G, Feyerabend S, Joung JY, Gravis G, Nishimura K, Gedye C, Padua C, Shore N, Thiery-Vuillemin A, Saad F, van Alphen R, Carducci MA, Desai C, Brickel N, Poehlein C, Del Rosario P, Fizazi K. Olaparib tolerability and common adverse-event management in patients with metastatic castration-resistant prostate cancer: Further analyses from the PROfound study. European journal of cancer (Oxford, England : 1990), 170:73-84, 2022
34. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, Lee KS, Niikura N, Park YH, Xu B, Wang X, Gil-Gil M, Li W, Pierga JY, Im SA, Moore HCF, Rugo HS, Yerushalmi R, Zagouri F, Gombos A, Kim SB, Liu Q, Luo T, Saura C, Schmid P, Sun T, Gambhire D, Yung L, Wang Y, Singh J, Vitazka P, Meinhardt G, Harbeck N, Cameron DA. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. The New England journal of medicine, 387:9-20, 2022
35. Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, Kawabata H, Masuda N, Futamura M, Minami H, Matsumoto K, Yoshimura K, Kitano S, Takano T. Safety and efficacy of nivolumab plus bevacizumab, paclitaxel for HER2-negative metastatic breast cancer: Primary results and biomarker data from a phase 2 trial (WJOG9917B). European journal of cancer (Oxford, England : 1990), 171:193-202, 2022
36. Niguma K, Mamishin K, Naito Y, Nomura S, Wakabayashi M, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Onishi T, Kawasaki T, Mukohara T. Impact of Older Age and Medico-social Factors on the Decision to Offer Adjuvant Chemotherapy to Patients With Breast Cancer. Anticancer research, 42:3743-3751, 2022
37. Kawahara T, Iwamoto T, Takashima I, Hanazawa R, Uemura K, Uemura Y, Mukai H, Kikawa Y, Taira N. Association of change in health-related quality of life and treatment discontinuation in metastatic breast cancer: a post hoc, exploratory analysis of two randomized clinical trials. Supportive care in cancer, 30:8367-8375, 2022
38. Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, Masuda N, Torregroza Otero M, Gokmen E, Loi S, Guo Z, Zhou X, Karantza V, Pan W, Schmid P. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. The New England journal of medicine, 387:217-226, 2022
39. Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, Kawabata H, Masuda N, Futamura M, Minami H, Matsumoto K, Yoshimura K, Kitano S, Takano T. Data of programmed death-ligand 1 expression and VEGF: Nivolumab, bevacizumab and paclitaxel For HER2-negative metastatic breast cancer. Data in brief, 45:108558, 2022
40. Narui K, Ishikawa T, Taira N, Uemura Y, Mukai H. Prospective Cohort Study of Palbociclib Treatment in Postmenopausal Patients With Unresectable and Metastatic Hormone Receptor-Positive Breast Cancer: Study Protocol for a CSPOR-BC Palbociclib Cohort Trial. World journal of oncology, 13:190-194, 2022
41. Miyahara K, Narui K, Uemura Y, Yamada A, Araki K, Fujisawa F, Nakayama T, Ishikawa T, Taira N, Kikawa Y, Aihara T, Mukai H. Prospective Cohort Study of Combination Therapy With Abemaciclib and Hormonal Therapy for Chemotherapy-Treated Patients With Hormone Receptor-Positive Metastatic Breast Cancer. World journal of oncology, 13:216-221, 2022
42. Geyer CE Jr, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, Rastogi P, Cui K, Arahmani A, Aktan G, Armstrong AC, Arnedos M, Balmaña J, Bergh J, Bliss J, Delaloge S, Domchek SM, Eisen A, Elsafy F, Fein LE, Fielding A, Ford JM, Friedman S, Gelmon KA, Gianni L, Gnant M, Hollingsworth SJ, Im SA, Jager A, Jóhannsson ÓÞ, Lakhani SR, Janni W, Linderholm B, Liu TW, Loman N, Korde L, Loibl S, Lucas PC, Marmé F, Martinez de Dueñas E, McConnell R, Phillips KA, Piccart M, Rossi G, Schmutzler R, Senkus E, Shao Z, Sharma P, Singer CF, Španić T, Stickeler E, Toi M, Traina TA, Viale G, Zoppoli G, Park YH, Yerushalmi R, Yang H, Pang D, Jung KH, Mailliez A, Fan Z, Tennevet I, Zhang J, Nagy T, Sonke GS, Sun Q, Parton M, Colleoni MA, Schmidt M, Brufsky AM, Razaq W, Kaufman B, Cameron D, Campbell C, Tutt ANJ. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Annals of oncology, 33:1250-1268, 2022
43. Shirai K, Guan G, Meihui T, Xiaoling P, Oka Y, Takahashi Y, Bhagat AAS, Yanagida M, Iwanaga S, Matsubara N, Mukohara T, Yoshida T. Hybrid double-spiral microfluidic chip for RBC-lysis-free enrichment of rare cells from whole blood. Lab on a chip, 22:4418-4429, 2022
44. Sawaki M, Taira N, Uemura Y, Saito T, Baba S, Kobayashi K, Kawashima H, Tsuneizumi M, Sagawa N, Bando H, Takahashi M, Yamaguchi M, Takashima T, Nakayama T, Kashiwaba M, Mizuno T, Yamamoto Y, Iwata H, Toyama T, Tsugawa K, Kawahara T, Mukai H. Adjuvant trastuzumab without chemotherapy for treating early HER2-positive breast cancer in older patients: A propensity score-adjusted analysis of a prospective cohort study. Breast (Edinburgh, Scotland), 66:245-254, 2022
45. Takahashi N, Kim S, Schultz CW, Rajapakse VN, Zhang Y, Redon CE, Fu H, Pongor L, Kumar S, Pommier Y, Aladjem MI, Thomas A. Replication stress defines distinct molecular subtypes across cancers. Cancer research communications, 2:503-517, 2022
46. Nishiga Y, Drainas AP, Baron M, Bhattacharya D, Barkal AA, Ahrari Y, Mancusi R, Ross JB, Takahashi N, Thomas A, Diehn M, Weissman IL, Graves EE, Sage J. Radiotherapy in combination with CD47 blockade elicits a macrophage-mediated abscopal effect. Nature cancer, 3:1351-1366, 2022
47. Uemoto Y, Yamanaka T, Kataoka Y, Wada Y, Aoyama Y, Kizawa R, Yamaguchi T, Kikawa Y, Mukai H, Taira N. Efficacy of Telemedicine Using Videoconferencing Systems in Outpatient Care for Patients With Cancer: A Systematic Review and Meta-Analysis. JCO clinical cancer informatics, 6:e2200084, 2022
48. Kikawa Y, Hagiwara Y, Fujisawa T, Araki K, Iwamoto T, Sangai T, Shien T, Takao S, Nishimura R, Takahashi M, Toyama T, Aihara T, Mukai H, Taira N. Health-related quality of life and estimation of the minimally important difference in the Functional Assessment of Cancer Therapy-Endocrine Symptom score in postmenopausal ER+/HER2- metastatic breast cancer with low sensitivity to endocrine therapy. PloS one, 17:e0278344, 2022
49. Abe Y, Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F, Shien T, Doihara H, Toyooka S. Association of Genetic Polymorphism with Taxane-induced Peripheral Neuropathy: Sub-analysis of a Randomized Phase II Study to Determine the Optimal Dose of 3-week Cycle Nab-Paclitaxel in Metastatic Breast Cancer Patients. Acta medica Okayama, 76:661-671, 2022
50. Fujita K, Suzuki H, Hinata N, Miura Y, Edamura K, Tabata KI, Arai G, Matsubara N, Yasumizu Y, Kosaka T, Oya M, Sugimoto M. Management of patients with advanced prostate cancer in Japan: ’real-world’ consideration of the results from the Advanced Prostate Cancer Consensus Conference. Translational andrology and urology, 11:1771-1785, 2022
51. Kuboki Y, Shimizu T, Yonemori K, Kojima T, Kondo S, Koganemaru S, Iwasa S, Harano K, Koyama T, Lu V, Zhou X, Niu H, Yanai T, Garcia-Ribas I, Doi T, Yamamoto N. Safety, Tolerability, and Pharmacokinetics of TAK-931, a Cell Division Cycle 7 Inhibitor, in Patients with Advanced Solid Tumors: A Phase I First-in-Human Study. Cancer research communications, 2:1426-1435, 2022
