Annual Report 2022
Department of Thoracic Oncology
Koichi Goto, Kiyotaka Yoh, Shingo Matsumoto, Yoshitaka Zenke, Kaname Nosaki, Hiroki Izumi, Yuji Shibata, Tetsuya Sakai, Shigeki Umemura, Hajime Oi, Masanobu Okahisa, Yosuke Kagawa, Yu Tanaka, Shingo Kitagawa, Shunta Mori, Yuji Uehara, Yu Ito
(Seiji Niho, Keisuke Kirita, Hibiki Udagawa, Eri Sugiyama)
Introduction
The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The department aims to provide the highest quality treatment and establish new effective treatments against lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance to our patients through multidisciplinary care, the staff members of the division work closely with thoracic surgeons, radiation oncologists, pathologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.

The Team and What We Do
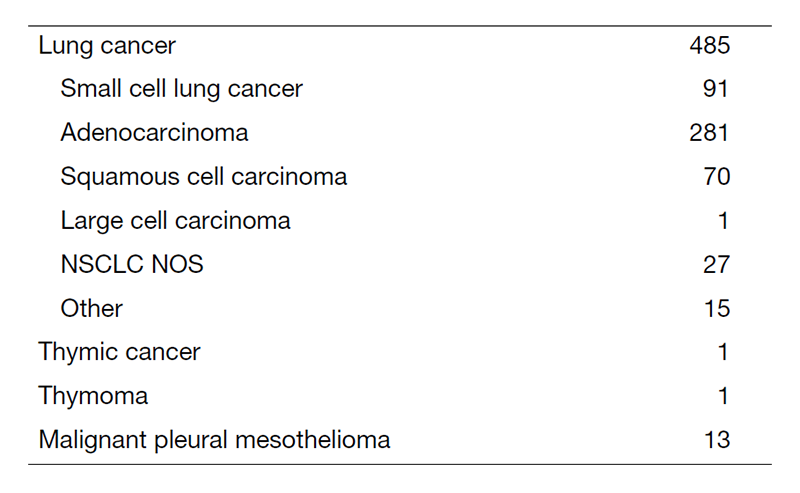
Our outpatient clinic, managed by the staff members and senior residents, is open from Monday to Friday to examine all new referred patients and evaluate returning patients. Returning patients also receive oral chemotherapy and/or intravenous chemotherapy in the Ambulatory Care Center. Bronchoscopy with EBUS for diagnosis is performed from Monday to Thursday afternoon. Fluoroscopic-CT guided needle lung biopsies are carried out on Tuesday afternoon. For patient management, we use approximately 50-60 beds mainly in 8F, 6A, 6B and 5A wards.
Case conferences on thoracic surgery and medical oncology are scheduled on Tuesday evenings and Wednesday evenings, respectively. The staff members and residents of the division participate in a journal club on Monday and Wednesday mornings. During monthly meetings with physicians in private hospitals, the staff members and residents teach methods of reading for chest X-ray and CT images.
Research Activities
Our research activities are focused on three areas: 1) development of new and effective diagnosis and treatment modalities in lung cancer; 2) collaborative studies with the Research Center for Innovative Oncology in the following areas: detection of biomarkers for the treatment of advanced lung cancer; development of new diagnostic methods of rare driver genomic alteration for lung cancer; correlation between genomic abnormalities and clinical characteristics and treatment in lung cancer; correlation between pathological features and sensitivity of treatments in lung cancer; and 3) translational research from bench to bedside or from bedside to bench for the development of innovative treatment strategies in lung cancer.
In particular, high-spec genomic analysis technologies are adapted to our research screening for rare driver genomic alterations of lung cancer such as RET, ROS1, BRAF, MET, and HER2 etc., and its clinical development is also supported in collaboration with some diagnostic companies.
Clinical Trials
The Department of Thoracic Oncology is currently conducting and participating in multi-institutional clinical studies for advanced lung cancer disease, such as the Japan Clinical Oncology Group (JCOG) trials, investigator-initiated trials, and pharmaceutical company-initiated global trials.
An Asian international genomic screening platform established by our department, whose name is LC-SCRUM-Asia, was initiated in 2013 and is now ongoing. As of February 2023, 186 Japanese institutions participated in LC-SCRUM-Japan, and 18,013 patients were enrolled. Furthermore, 5 institutions in Taiwan participated in our genomic screening between March 2019 and February 2022, and 394 patients were enrolled from Taiwan. LC-SCRUM-Asia will support the development of novel therapeutic and diagnostic products and contribute to the establishment of precision medicine in Asian countries. Many lung cancers with rare driver oncogenes, such as RET, ROS1 BRAF, MET, HER2 NTRK and KRAS G12C genomic alterations were identified in our screening and entered into various clinical trials of molecular targeting agents. Based on the results of clinical trials leveraging genomic screening in LC-SCRUM-Asia, crizotinib was approved for ROS1 fusion positive lung cancer in May 2017, dabrafenib/trametinib was approved for BRAF V600E mutation positive lung cancer in March 2018, entrectinib was approved for NTRK and ROS1 fusion positive lung cancer in June 2019 and February 2020, respectively, and tepotinib and capmatinib were approved for MET ex14 skipping positive lung cancer in March 2020 and June 2020, respectively. In addition, selpercatinib was approved for RET fusion positive lung cancer in September 2021 and sotorasib was approved for KRAS G12C mutation positive lung cancer in January 2022.
The RT-PCR kit, which was adapted for LC-SCRUM-Asia screening for ROS1 fusion, was simultaneously approved using our screening data as a companion diagnostic (CDx) for ROS1-positive lung cancer in January 2017. In the same way, the next-generation sequencing (NGS) panel was first approved as a CDx for BRAF mutation-positive lung cancer in April 2018 and was also approved as the first multi-NGS CDx for EGFR/ALK/ROS1/BRAF in February 2019. In this year, a multi-PCR panel with short turnaround time was approved as a multi-CDx for EGFR/ALK/ROS1/BRAF/MET/RET/KRAS in March 2023. As a result of this, we can obtain the results of multi genomic analysis only in a week and start precision medicine for lung cancer from first-line treatment in clinical practice. Through genomic screening and the established clinical-genomic database, LC-SCRUM-Asia can play a key role in the development of precision medicine in lung cancer in Japan and Asian countries.
In addition to the targeted genomic screening, to identify some novel oncogenic driver genes, we initiated whole-transcriptome sequencing (WTS) of NSCLC samples negative for known oncogenic drivers in LC-SCRUM-Asia from October 2020. In the WTS, we identified an in-frame fusion transcript of CLIP1 on chromosome 12q24 and LTK on chromosome 15q15 in one patient. The CLIP1-LTK fusion was present in 0.4% of NSCLCs and was mutually exclusive with other known oncogenic drivers. We showed that the kinase activity of the CLIP1-LTK fusion protein was constitutively activated and had transformation potential. In vitro and in vivo analyses showed that treatment with lorlatinib, an ALK inhibitor, inhibited CLIP1-LTK kinase activity, suppressed proliferation and induced apoptosis. One patient with NSCLC harboring the CLIP1-LTK fusion showed a good clinical response to lorlatinib. This was the first description of LTK alterations with oncogenic activity in cancers and was published in Nature in November 2021. Moreover, we started an investigator-initiated trial of lorlatinib for CLIP1-LTK fusion-positive lung cancer in February 2023.
We also initiated an additional genomic screening project for pre-treated patients to identify resistant genomic alterations after treatment with molecular targeting agents (LC-SCRUM-TRY) from September 2020. A total of 1,329 pre-treated patients were already enrolled in LC-SCRUM-TRY as of February 2023. We challenge to establish precision medicine according to the resistance mechanism to previous treatment by leveraging genomic screening of LC-SCRUM-TRY. Moreover, we started a new genomic screening project (LC-SCRUM-Advantage/MRD) for patients with stage I-III lung cancers who underwent surgical resection from August 2022 to establish precision medicine during perioperative treatment and clarify the clinical significance of micro residual disease (MRD) in blood samples.
To select optimal treatment for individual patients with advanced lung cancer, we currently need to identify genomic alterations by multiple genomic tests and perform PD-L1 immunohistochemical staining using tissue samples in clinical practice. Since we need to obtain large amount of tissue samples as possible by bronchoscopy for the biomarker analyses, we are conducting a feasibility study of the cutting-edge trans-bronchial biopsy technique to evaluate its utility.
Education
Residents, cancer specialists in training, and staff are paired to provide outpatient/inpatient medical care and perform examinations. The aim is to develop clinicians who can provide comprehensive medical care for patients with thoracic malignant tumors, from diagnosis to treatment, including palliative care, by closely supporting patients with advanced lung cancer for whom a complete cure is difficult. Moreover, in our department, we always try to train specialists who not only possess outstanding minds but also strong mental and physical strengths to handle patients’ distress. Residents are required to go on rotation in the Department of Pathology during their training period, and have opportunities to be involved with basic research from this department. Furthermore, we also actively support the preparation of manuscripts on basic and clinical research, aiming to develop clinicians capable of conducting clinical and translational research. In addition, one resident is affiliated with the Joint Graduate Program at Juntendo University and Jikei University, and two specialized researchers have been studying at Georgetown University in the United States since September 2018 and MD Anderson Cancer Center in the United States since October 2020, respectively.
A joint case conference with the Department of Thoracic Surgery is held every Tuesday, a joint case conference with the Department of Radiation Oncology is held every Wednesday to determine treatment policies, and a research conference is held once a month to discuss the progress and schedule of research with all members. A journal club with the Department of Thoracic Oncology is held every Monday, a journal club with the Department of Thoracic Surgery is held every Wednesday, a joint conference with the Departments of Thoracic Surgery and Pathology is held every Friday, a chest X-ray reading meeting for local clinicians is held on the second Tuesday of each month, and a chest X-ray reading meeting with the Katsushika Medical Association is held on the fourth Tuesday of each month.
Future Prospects
Drug therapy for lung cancer has made a major shift toward precision medicine in which therapeutic drugs are selected based on biomarkers in individual patients. Furthermore, immune checkpoint inhibitors have greatly contributed to the improvement of treatment outcomes in lung cancer as these are highly effective drugs with a mechanism of action different from that of conventional drugs. Dramatic advances in these new therapies have greatly changed the treatment landscape for advanced lung cancer in the last few years, and treatment outcomes have also significantly improved. In our department, we plan to continue conducting various research studies in the future with the aim of establishing precision medicine for lung cancer based on biomarkers. Additionally Asian countries such as Thailand, Malaysia and Taiwan are taking part in LC-SCRUM-Asia, and in collaboration with genomic screening in China, we would like to establish a large-scale international genomic screening platform in Asian countries. By leveraging this screening platform, treatment development for advanced lung cancer, which has attracted a great deal of attention worldwide, will be conducted and the results will be released to the world. Moreover, we will continue to promote translational research, conduct innovative and advanced clinical trials that can link the results to clinical development, and pursue research with the aim of overcoming advanced lung cancer.
List of papers published in 2022
Journal
1. Udagawa H, Takahashi S, Hirao M, Tahara M, Iwasa S, Sato Y, Hamakawa T, Shitara K, Horinouchi H, Chin K, Masuda N, Suzuki T, Okumura S, Takase T, Nagai R, Yonemori K. Liposomal eribulin for advanced adenoid cystic carcinoma, gastric cancer, esophageal cancer, and small cell lung cancer. Cancer medicine, 12:1269-1278, 2023
2. Noda-Narita S, Naito T, Udagawa H, Goto K, Miyawaki T, Mamesaya N, Nakashima K, Kenmotsu H, Shimokawaji T, Kato T, Hakozaki T, Okuma Y, Nakamura M, Nakayama Y, Watanabe H, Kusumoto M, Ohe Y, Horinouchi H. Nivolumab-induced radiation recall pneumonitis in non-small-cell lung cancer patients with thoracic radiation therapy. Cancer science, 114:630-639, 2023
3. Goto Y, Yoh K, Kato T, Hosomi Y, Usui K, Fukui T, Hirano K, Tanaka H, Taguri M, Kunitoh H. Observational study to predict the efficacy and optimal duration of nivolumab treatment in patients with previously treated advanced or recurrent non-small cell lung cancer. Japanese journal of clinical oncology, 53:153-160, 2023
4. Goto K, Shiraishi Y, Murakami H, Horinouchi H, Toyozawa R, Takeda M, Uno M, Crawford N, McGill J, Jimbo T, Ishigami M, Takayama G, Nakayama S, Ohwada S, Nishio M. Phase 1 study of DS-1205c combined with gefitinib for EGFR mutation-positive non-small cell lung cancer. Cancer medicine, 12:7090-7104, 2023
5. Paz-Ares L, Champiat S, Lai WV, Izumi H, Govindan R, Boyer M, Hummel HD, Borghaei H, Johnson ML, Steeghs N, Blackhall F, Dowlati A, Reguart N, Yoshida T, He K, Gadgeel SM, Felip E, Zhang Y, Pati A, Minocha M, Mukherjee S, Goldrick A, Nagorsen D, Hashemi Sadraei N, Owonikoko TK. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. Journal of clinical oncology, 41:2893-2903, 2023
6. Fujiwara Y, Makihara R, Hase T, Hashimoto N, Naito T, Tsubata Y, Okuno T, Takahashi T, Kobayashi H, Shinno Y, Zenke Y, Ikeda T, Hosomi Y, Watanabe K, Kitazono S, Sakiyama N, Makino Y, Yamamoto N. Pharmacokinetic and dose-finding study of osimertinib in patients with impaired renal function and low body weight. Cancer science, 114:2087-2097, 2023
7. Doi T, Shitara K, Kojima T, Kuboki Y, Matsubara N, Bando H, Yoh K, Naito Y, Hirai H, Kurokawa Y, Kato T, Morizane C. Phase I study of the irreversible fibroblast growth factor receptor 1-4 inhibitor futibatinib in Japanese patients with advanced solid tumors. Cancer science, 114:574-585, 2023
8. Sakai T, Aokage K, Miyoshi T, Tane K, Ishii G, Goto K, Tsuboi M. Tumor size exceeding 5 cm as a valid prognostic factor in all stages of thymic epithelial tumors. Surgery today, 53:42-50, 2023
9. Drilon A, Subbiah V, Gautschi O, Tomasini P, de Braud F, Solomon BJ, Shao-Weng Tan D, Alonso G, Wolf J, Park K, Goto K, Soldatenkova V, Szymczak S, Barker SS, Puri T, Bence Lin A, Loong H, Besse B. Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. Journal of clinical oncology, 41:385-394, 2023
10. Sakai T, Aokage K, Miyoshi T, Tane K, Ishii G, Goto K, Tsuboi M. Correction to: Tumor size exceeding 5 cm as a valid prognostic factor in all stages of thymic epithelial tumors. Surgery today, 53:51, 2023
11. Sugimoto A, Matsumoto S, Udagawa H, Itotani R, Usui Y, Umemura S, Nishino K, Nakachi I, Kuyama S, Daga H, Hara S, Miyamoto S, Kato T, Sakakibara-Konishi J, Tabata E, Nakagawa T, Kawaguchi T, Sakai T, Shibata Y, Izumi H, Nosaki K, Zenke Y, Yoh K, Goto K. A Large-Scale Prospective Concordance Study of Plasma- and Tissue-Based Next-Generation Targeted Sequencing for Advanced Non-Small Cell Lung Cancer (LC-SCRUM-Liquid). Clinical cancer research, 29:1506-1514, 2023
12. Tamiya Y, Matsumoto S, Zenke Y, Yoh K, Ikeda T, Shibata Y, Kato T, Nishino K, Nakamura A, Furuya N, Miyamoto S, Kuyama S, Nomura S, Ikeno T, Udagawa H, Sugiyama E, Nosaki K, Izumi H, Sakai T, Hashimoto N, Goto K. Large-scale clinico-genomic profile of non-small cell lung cancer with KRAS G12C: Results from LC-SCRUM-Asia study. Lung cancer (Amsterdam, Netherlands), 176:103-111, 2023
13. Takeda M, Shimokawa M, Nakamura A, Nosaki K, Watanabe Y, Kato T, Hayakawa D, Tanaka H, Takahashi T, Oki M, Tachihara M, Fujimoto D, Hayashi H, Yamaguchi K, Yamamoto S, Iwama E, Azuma K, Hasegawa K, Yamamoto N, Nakagawa K. A phase II study (WJOG12819L) to assess the efficacy of osimertinib in patients with EGFR mutation-positive NSCLC in whom systemic disease (T790M-negative) progressed after treatment with first- or second-generation EGFR TKIs and platinum-based chemotherapy. Lung cancer (Amsterdam, Netherlands), 177:44-50, 2023
14. Tanaka Y, Nakai T, Suzuki A, Kagawa Y, Noritake O, Taki T, Hashimoto H, Sakai T, Shibata Y, Izumi H, Nosaki K, Udagawa H, Zenke Y, Matsumoto S, Yoh K, Miyazaki S, Sakamoto N, Sakashita S, Kojima M, Watanbe R, Tsuboi M, Goto K, Ishii G. Clinicopathological significance of peritumoral alveolar macrophages in patients with resected early-stage lung squamous cell carcinoma. Cancer immunology, immunotherapy, 72:2205-2215, 2023
15. Kagawa Y, Hayashida T, Liu J, Mori S, Izumi H, Kumagai S, Udagawa H, Hattori N, Goto K, Kobayashi SS. The EGFR C797S Mutation Confers Resistance to a Novel EGFR Inhibitor CLN-081 to EGFR Exon 20 Insertion Mutations. JTO clinical and research reports, 4:100462, 2023
16. Shiraishi T, Yamasaki K, Kidogawa M, Shingu T, Ujimiya F, Jotatsu T, Matsumoto S, Izumi H, Nishida C, Goto K, Yatera K. Successful Treatment with Crizotinib to Overcome Drug Resistance Possibly Due to Mesenchymal-epithelial Transition Amplification in a Lung Cancer Patient with the Echinoderm Microtubule-associated Protein-like 4-anaplastic Lymphoma Kinase Fusion Gene. Internal medicine (Tokyo, Japan), 2023
17. Aokage K, Tsuboi M, Zenke Y, Horinouchi H, Nakamura N, Ishikura S, Nishikawa H, Kumagai S, Koyama S, Kanato K, Kataoka T, Wakabayashi M, Fukutani M, Fukuda H, Ohe Y, Watanabe SI. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non-small cell lung cancer. Japanese journal of clinical oncology, 52:383-387, 2022
18. Matsumoto Y, Umemura S, Okizaki A, Fujisawa D, Kobayashi N, Tanaka Y, Sasaki C, Shimizu K, Ogawa A, Kinoshita H, Uchitomi Y, Yoshiuchi K, Matsuyama Y, Morita T, Goto K, Ohe Y. Early specialized palliative care for patients with metastatic lung cancer receiving chemotherapy: a feasibility study of a nurse-led screening-triggered programme. Japanese journal of clinical oncology, 52:375-382, 2022
19. Ohe Y, Yamazaki N, Yamamoto N, Murakami H, Yoh K, Kitano S, Hashimoto H, Murayama A, Nakane S, Gemma A. The real-world safety of atezolizumab as second-line or later treatment in Japanese patients with non-small-cell lung cancer: a post-marketing surveillance study. Japanese journal of clinical oncology, 52:623-632, 2022
20. Shukuya T, Takamochi K, Sakurai H, Yoh K, Hishida T, Tsuboi M, Goto Y, Kudo Y, Ohde Y, Okumura S, Taguri M, Kunitoh H. Efficacy of Adjuvant Chemotherapy With Tegafur-Uracil in Patients With Completely Resected, Node-Negative NSCLC-Real-World Data in the Era of Molecularly Targeted Agents and Immunotherapy. JTO clinical and research reports, 3:100320, 2022
21. Zenke Y, Hakozaki T, Nakahara Y, Horinouchi H, Ohe Y. Medical management of older patients with lung cancer. Japanese journal of clinical oncology, 52:1082-1088, 2022
22. Sugawara S, Kondo M, Yokoyama T, Kumagai T, Nishio M, Goto K, Nakagawa K, Seto T, Yamamoto N, Kudou K, Asato T, Zhang P, Ohe Y. Brigatinib in Japanese patients with tyrosine kinase inhibitor-naive ALK-positive non-small cell lung cancer: first results from the phase 2 J-ALTA study. International journal of clinical oncology, 27:1828-1838, 2022
23. Hosomi Y, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Tajima K, Kajihara Y, Yamamoto N. Erlotinib with or without bevacizumab as a first-line therapy for patients with advanced nonsquamous epidermal growth factor receptor-positive non-small cell lung cancer: Exploratory subgroup analyses from the phase II JO25567 study. Thoracic cancer, 13:2192-2200, 2022
24. Wu YL, Lu S, Yang JC, Zhou J, Seto T, Ahn MJ, Su WC, Yamamoto N, Kim DW, Paolini J, Usari T, Iadeluca L, Wilner KD, Goto K. Final Overall Survival, Safety, and Quality of Life Results From a Phase 2 Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced NSCLC. JTO clinical and research reports, 3:100406, 2022
25. Takamochi K, Suzuki K, Tsuboi M, Niho S, Ishikura S, Oyamada S, Yamaguchi T, Okada M. Randomized phase II trial of pemetrexed-cisplatin plus bevacizumab or thoracic radiotherapy followed by surgery for stage IIIA (N2) nonsquamous non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery, 164:661-671.e4, 2022
26. Demetri GD, De Braud F, Drilon A, Siena S, Patel MR, Cho BC, Liu SV, Ahn MJ, Chiu CH, Lin JJ, Goto K, Lee J, Bazhenova L, John T, Fakih M, Chawla SP, Dziadziuszko R, Seto T, Heinzmann S, Pitcher B, Chen D, Wilson TR, Rolfo C. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Patients With NTRK Fusion-Positive Solid Tumors. Clinical cancer research, 28:1302-1312, 2022
27. McCoach CE, Rolfo C, Drilon A, Lacouture M, Besse B, Goto K, Zhu VW, Tan DSW, Farajian S, Potter LA, Kherani JF, Soldatenkova V, Olek EA, Muehlenbein CE, Park K. Hypersensitivity Reactions to Selpercatinib Treatment With or Without Prior Immune Checkpoint Inhibitor Therapy in Patients With NSCLC in LIBRETTO-001. Journal of thoracic oncology, 17:768-778, 2022
28. Kunimasa K, Matsumoto S, Nishino K, Honma K, Maeda N, Kuhara H, Tamiya M, Inoue T, Kawamura T, Kimura T, Maniwa T, Okami J, Goto K, Kumagai T. Comparison of sampling methods for next generation sequencing for patients with lung cancer. Cancer medicine, 11:2744-2754, 2022
29. Remon J, Lacas B, Herbst R, Reck M, Garon EB, Scagliotti GV, Ramlau R, Hanna N, Vansteenkiste J, Yoh K, Groen HJM, Heymach JV, Mandrekar SJ, Okamoto I, Neal JW, Heist RS, Planchard D, Pignon JP, Besse B. ANtiangiogenic Second-line Lung cancer Meta-Analysis on individual patient data in non-small cell lung cancer: ANSELMA. European journal of cancer (Oxford, England : 1990), 166:112-125, 2022
30. Kenmotsu H, Wakuda K, Mori K, Kato T, Sugawara S, Kirita K, Yoneshima Y, Azuma K, Nishino K, Teraoka S, Shukuya T, Masuda K, Hayashi H, Toyozawa R, Miura S, Fujimoto D, Nakagawa K, Yamamoto N, Takahashi T. Randomized Phase 2 Study of Osimertinib Plus Bevacizumab Versus Osimertinib for Untreated Patients With Nonsquamous NSCLC Harboring EGFR Mutations: WJOG9717L Study. Journal of thoracic oncology, 17:1098-1108, 2022
31. Aizawa R, Nakamura Y, Ikeda T, Aibara N, Kutsuna YJ, Kurosaki T, Aki K, Junya H, Nakagawa H, Sato K, Kodama Y, Nakashima MN, Nakashima M, Mukae H, Ohyama K. Immune complexome analysis of serum samples from non-small-cell lung cancer patients identifies predictive biomarkers for nivolumab therapy. Clinica chimica acta; international journal of clinical chemistry, 532:84-88, 2022
32. Nishio M, Ohe Y, Ohashi K, Toyozawa R, Satouchi M, Sekine N, Mori J, Enatsu S, Goto K. [Efficacy and Safety Analysis of Selpercatinib in Patients with RET Fusion-Positive Non-Small Cell Lung Cancer-Results from the Japanese Subset of a Global Phase 1/2 Study]. Gan to kagaku ryoho. Cancer & chemotherapy, 49:669-675, 2022
33. Hotta K, Hida T, Nokihara H, Morise M, Kim YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Kumagai T, Watanabe S, Goto K, Satouchi M, Kozuki T, Shukuya T, Nakagawa K, Mitsudomi T, Yamamoto N, Asakawa T, Yoshimoto T, Takata S, Tamura T. Final overall survival analysis from the phase III J-ALEX study of alectinib versus crizotinib in ALK inhibitor-naïve Japanese patients with ALK-positive non-small-cell lung cancer. ESMO open, 7:100527, 2022
34. He Y, Kim IK, Bian J, Polyzos A, Di Giammartino DC, Zhang YW, Luo J, Hernandez MO, Kedei N, Cam M, Borczuk AC, Lee T, Han Y, Conner EA, Wong M, Tillo DC, Umemura S, Chen V, Ruan L, White JB, Miranda IC, Awasthi PP, Altorki NK, Divakar P, Elemento O, Apostolou E, Giaccone G. A Knock-In Mouse Model of Thymoma With the GTF2I L424H Mutation. Journal of thoracic oncology, 17:1375-1386, 2022
35. Hasegawa T, Okuyama T, Uemura T, Matsuda Y, Otani H, Shimizu J, Horio Y, Watanabe N, Yamaguchi T, Fukuda S, Oguri T, Maeno K, Tamiya A, Nosaki K, Fukumitsu K, Akechi T. Prognostic Awareness and Discussions of Incurability in Patients with Pretreated Non-Small Cell Lung Cancer and Caregivers: A Prospective Cohort Study. The oncologist, 27:982-990, 2022
36. Takamochi K, Tsuboi M, Okada M, Niho S, Ishikura S, Oyamada S, Yamaguchi T, Suzuki K. S-1 + Cisplatin with Concurrent Radiotherapy Followed by Surgery for Stage IIIA (N2) Lung Squamous Cell Carcinoma: Results of a Phase II Trial. Annals of surgical oncology, 29:8198-8206, 2022
37. Takamochi K, Tsuboi M, Okada M, Niho S, Ishikura S, Oyamada S, Yamaguchi T, Suzuki K. ASO Visual Abstract: S-1 + Cisplatin with Concurrent Radiotherapy Followed by Surgery for Stage IIIA (N2) Lung Squamous Cell Carcinoma: Results of a Phase II Trial. Annals of surgical oncology, 29:8209-8210, 2022
38. Sugawara S, Kondo M, Yokoyama T, Kumagai T, Nishio M, Goto K, Nakagawa K, Seto T, Yamamoto N, Kudou K, Asato T, Zhang P, Ohe Y. Correction to: Brigatinib in Japanese patients with tyrosine kinase inhibitor-naive ALK-positive non-small cell lung cancer: first results from the phase 2 J-ALTA study. International journal of clinical oncology, 27:1839-1840, 2022
39. Tamiya Y, Nakai T, Suzuki A, Mimaki S, Tsuchihara K, Sato K, Yoh K, Matsumoto S, Zenke Y, Nosaki K, Izumi H, Shibata Y, Sakai T, Taki T, Miyazaki S, Watanabe R, Sakamoto N, Sakashita S, Kojima M, Hashimoto N, Tsuboi M, Goto K, Ishii G. The impact of tertiary lymphoid structures on clinicopathological, genetic and gene expression characteristics in lung adenocarcinoma. Lung cancer (Amsterdam, Netherlands), 174:125-132, 2022
40. Nilsson MB, Yang Y, Heeke S, Patel SA, Poteete A, Udagawa H, Elamin YY, Moran CA, Kashima Y, Arumugam T, Yu X, Ren X, Diao L, Shen L, Wang Q, Zhang M, Robichaux JP, Shi C, Pfeil AN, Tran H, Gibbons DL, Bock J, Wang J, Minna JD, Kobayashi SS, Le X, Heymach JV. CD70 is a therapeutic target upregulated in EMT-associated EGFR tyrosine kinase inhibitor resistance. Cancer Cell, 41:340-355.e346, 2023
