Annual Report 2022
Department of Hepatobiliary and Pancreatic Surgery
Naoto Gotohda, Shin Kobayashi, Motokazu Sugimoto, Masashi Kudo, Masaru Konishi, Yusuke Abe, Tatsuki Ishikawa, Kimimasa Sasaki, Yu Igata, Norikazu Une, Taiki Sunakawa, Keishiro Aoki, Masaaki Kagoura, Tsuyoshi Terada
Introduction
The Department of Hepatobiliary and Pancreatic Surgery consists of five staff surgeons, three chief residents and six residents. Our department is responsible for the surgical treatment of patients with hepatic, biliary, pancreatic and duodenal cancer or low-grade malignant tumors. We conduct multidisciplinary treatment in cooperation with the Department of Hepatobiliary and Pancreatic Oncology, the Department of Diagnostic Radiology, and the Department of Radiation Oncology. In addition to conventional open surgery, we perform minimally invasive surgery including laparoscopic and robot-assisted surgery for patients with liver and pancreatic tumors.
The Team and What We Do
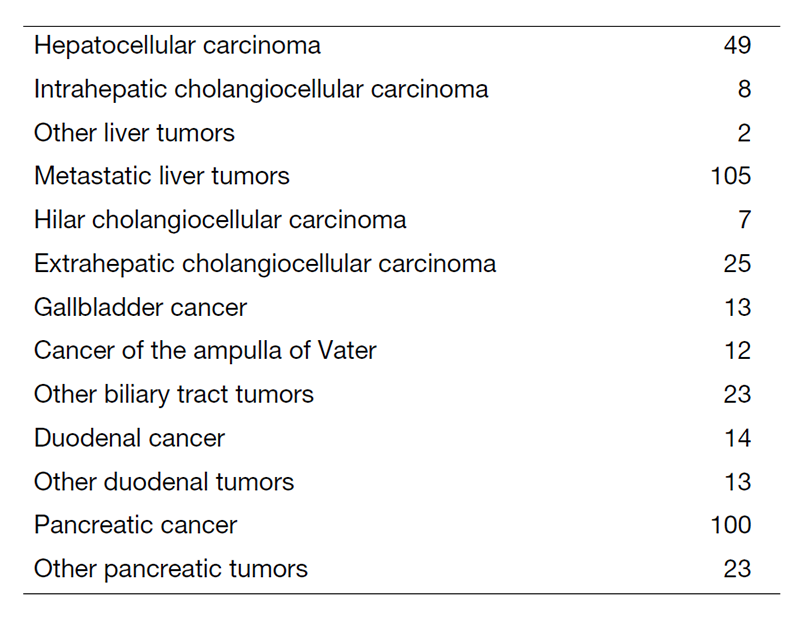
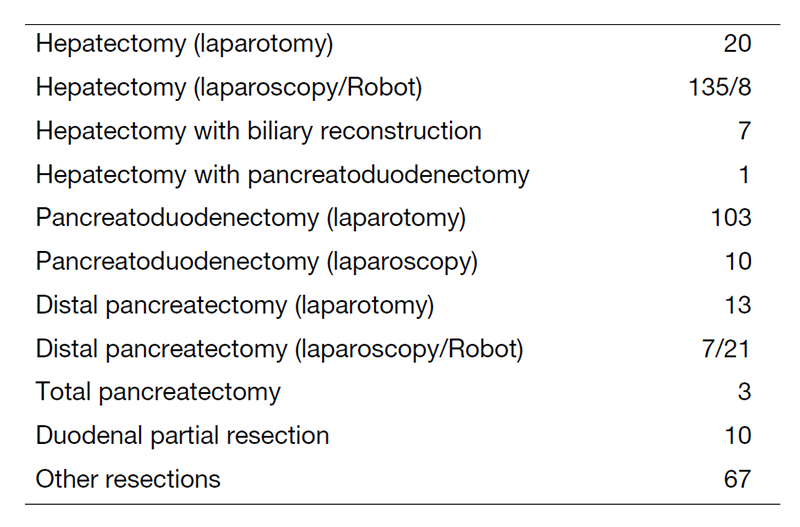
We work with outpatients five days a week and have approximately 25 inpatients. Staff meetings in which we discuss the treatment strategy or the key points of surgery for patients are held with all members of the department every morning. A Cancer Board is held in cooperation with radiologists and medical oncologists every Tuesday. A pathology conference is held monthly with pathologists. In 2022, 417 patients with hepatobiliary and pancreatic diseases underwent surgical treatment. The main diseases are listed in Table 1. Compared to the number of patients in 2021, there was an increase in the utilization of minimally invasive liver surgery (Table 2). We performed many laparoscopic surgeries for liver and pancreatic tumors in 2022. Furthermore, we introduced robot-assisted liver surgery and distal pancreatectomy into our practice in 2022.
Research Activities
1. Conversion surgery for pancreatic cancer
Currently, the treatment outcomes of pancreatic cancer patients are improving remarkably with the chemotherapy regimen Gem+nab-PTX or FOLFIRINOX. We attempt conversion surgery for selected patients with borderline resectable or unresectable pancreatic cancer who received chemotherapy. We aim to determine the most appropriate duration of chemotherapy and identify good indications for conversion surgery.
2. Function-preserving surgery
Pancreas-sparing duodenectomy (PSD) represents an alternative procedure to pancreaticoduodenectomy (PD) for patients with duodenal neoplasms. PSD is a function-preserving surgery technique and has the advantage over PD of preservation of the pancreas. We are attempting to establish a safe procedure for PSD.
3. Evaluation of liver function
Postoperative liver failure is one of the fatal complications after major hepatectomy. In our practice, we assess liver function using the indocyanine green retention rate at 15 min (ICG15) test. We are developing an alternative method to evaluate liver function employing liver-specific magnetic resonance imaging (MRI) with a gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) contrast agent instead of using the ICG15 test.
4. Cancer genome screening
We conducted clinical studies for cancer genome screening. For patients with resectable HCC, analysis by blood screening (COSMOS-HCC-01) was started in 2020.
“A multicenter proof of concept study for personalized perioperative therapy based on genetic alteration status for resectable oligometastases from colorectal cancer” (PRECISION study) (UMIN000042490): This is a multicenter prospective observational study to investigate the clinical utility of pre-treatment ctDNA analysis in patients undergoing surgery for colorectal oligometastases, conducted with a grant from AMED (20ck0106629h0001). The main eligibility criterion is patients with previously untreated resectable colorectal oligometastases, and the primary endpoint is the detection rate of pre-treatment ctDNA and the positive rate of BRAF V600E in tissue. The period of accrual and follow-up is two years each, and the study started in March 2021.
Clinical Trials
1. A phase III trial of S-1 versus observation in patients with resected biliary tract cancer (JCOG1202). Recruitment was completed in 2018, and the primary endpoint was analyzed in 2021, with the result published in the Lancet in January 2023. The three-year survival rate was 67.6% in the surgery group, and 77.1% in the adjuvant chemotherapy group, showing a significant difference between the two groups. Based on these results, adjuvant chemotherapy with S-1 is now considered as the standard treatment for biliary tract cancer.
2. A non-randomized controlled study comparing proton beam therapy and hepatectomy for resectable hepatocellular carcinoma (JCOG1315C). Recruitment started in 2016.
3. Japanese trial - A global study to evaluate the potential benefit of adjuvant chemotherapy for small bowel adenocarcinoma (JCOG1502C). Recruitment started in 2017.
4. A randomized phase II/III study of gemcitabine and nab-paclitaxel therapy versus S-1 and concurrent radiotherapy as neoadjuvant treatment for borderline resectable pancreatic cancer (GABARNANCE Trial). Recruitment was completed in 2022
5. A randomized phase III trial of neoadjuvant gemcitabine and cisplatin and S-1 (GCS) versus surgery first for resectable biliary tract cancer (JCOG1920). Recruitment was started in 2021.
6. A randomized phase III trial of neoadjuvant gemcitabine and S-1 (GS) versus gemcitabine and nab-paclitaxel (GnP) for resectable pancreatic cancer in geriatric patients (JCOG2101C). Recruitment started in 2022.
Education
“Board-certified expert surgeons” have a high level of skill in the field of hepatobiliary-pancreatic surgery. To qualify, surgeons must perform a specified number of operations under the guidance of a board-certified instructor. The residents in our department are being trained to receive the certification by the end of the chief resident course.
Future Prospects
Our goal is the establishment of multidisciplinary treatment for patients with refractory hepatobiliary and pancreatic cancer and the establishment of minimally invasive surgery for patients with pancreatic cancer and liver cancer. We are conducting robot-assisted surgeries in our field.
List of papers published in 2022
Journal
1. Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, Todaka A, Yanagimoto H, Morinaga S, Kobayashi S, Shimada K, Takahashi Y, Nakagohri T, Gotoh K, Kamata K, Shimizu Y, Ueno M, Ishii H, Okusaka T, Furuse J. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet (London, England), 401:195-203, 2023
2. Sasaki K, Ito M, Kobayashi S, Kitaguchi D, Matsuzaki H, Kudo M, Hasegawa H, Takeshita N, Sugimoto M, Mitsunaga S, Gotohda N. Automated surgical workflow identification by artificial intelligence in laparoscopic hepatectomy: Experimental research. International journal of surgery (London, England), 105:106856, 2022
3. Takahashi S, Ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T, Kojima M, Morinaga S, Toyama H, Shimizu Y, Miyamoto A, Tomikawa M, Takakura N, Takayama W, Hirano S, Otsubo T, Nagino M, Kimura W, Sugimachi K, Uesaka K. Neoadjuvant S-1 With Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-label Multicenter Prospective Trial (JASPAC05). Annals of surgery, 276:e510-e517, 2022
4. Kudo M, Gotohda N, Sugimoto M, Konishi M, Takahashi S, Kobayashi S, Kobayashi T. The Assessment of Regional Liver Function Before Major Hepatectomy Using Magnetic Resonance Imaging. The American surgeon, 88:2353-2360, 2022
5. Okamura Y, Boku N, Ghaneh P, Greenhalf W, Yasukawa S, Narimatsu H, Fukutomi A, Konishi M, Morinaga S, Toyama H, Maeda A, Shimizu Y, Nakamori S, Sata N, Yamakita K, Takahashi A, Takayama W, Yamaguchi R, Tomikawa M, Yanagisawa A, Neoptolemos JP, Uesaka K. Concordance of human equilibrative nucleoside transporter-1 expressions between murine (10D7G2) and rabbit (SP120) antibodies and association with clinical outcomes of adjuvant chemotherapy for pancreatic cancer: A collaborative study from the JASPAC 01 trial. Cancer reports (Hoboken, N.J.), 5:e1507, 2022
6. Yagi N, Suzuki T, Mizuno S, Kojima M, Kudo M, Sugimoto M, Kobayashi S, Gotohda N, Ishii G, Nakatsura T. Component with abundant immune-related cells in combined hepatocellular cholangiocarcinoma identified by cluster analysis. Cancer science, 113:1564-1574, 2022
7. Kudo M, Ishii G, Gotohda N, Konishi M, Takahashi S, Kobayashi S, Sugimoto M, Martin JD, Cabral H, Kojima M. Histological tumor necrosis in pancreatic cancer after neoadjuvant therapy. Oncology reports, 48:121, 2022
8. Sugimoto M, Gotohda N, Kudo M, Kobayashi S, Takahashi S, Konishi M. Laparoscopic liver resection can be performed safely without intraoperative drain placement. Surgical endoscopy, 36:9019-9031, 2022
9. Morise Z, Aldrighetti L, Belli G, Ratti F, Cheung TT, Lo CM, Tanaka S, Kubo S, Okamura Y, Uesaka K, Monden K, Sadamori H, Hashida K, Kawamoto K, Gotohda N, Chen K, Kanazawa A, Takeda Y, Ohmura Y, Ueno M, Ogura T, Suh KS, Kato Y, Sugioka A, Belli A, Nitta H, Yasunaga M, Cherqui D, Halim NA, Laurent A, Kaneko H, Otsuka Y, Kim KH, Cho HD, Lin CC, Ome Y, Seyama Y, Troisi RI, Berardi G, Rotellar F, Wilson GC, Geller DA, Soubrane O, Yoh T, Kaizu T, Kumamoto Y, Han HS, Ekmekcigil E, Dagher I, Fuks D, Gayet B, Buell JF, Ciria R, Briceno J, O’Rourke N, Lewin J, Edwin B, Shinoda M, Abe Y, Hilal MA, Alzoubi M, Tanabe M, Wakabayashi G. An International Retrospective Observational Study of Liver Functional Deterioration after Repeat Liver Resection for Patients with Hepatocellular Carcinoma. Cancers, 14:2598, 2022
10. Yasuta S, Sugimoto M, Kudo M, Kobayashi S, Takahashi S, Konishi M, Gotohda N. Early postoperative decrease of skeletal muscle mass predicts recurrence and poor survival after surgical resection for perihilar cholangiocarcinoma. BMC cancer, 22:1358, 2022
