Annual Report 2022
CLINICAL RESEARCH SUPPORT OFFICE
- Clinical Research Planning Division
Nozomu Fuse
- Clinical Research Management Division
Clinical Trial Management Section
Keiko Kobayashi, Hiromi Ono, Hitomi Tamura, Makoto Fukui, Yuichi Mikamoto, Mitiko Suzuki, Kozi Takahasi, Maiko Takakusa, Yukiko Ishiguro, Masatoshi Asano, Hidekazu Furuya, Makiko Makishima, Ayako Konishi, Yu Komura, Hitomi Kubota, Rie Shinohara, Kaori Terauchi, Yuuko Inakodani, Chiaki Kurihara, Kazuo Shibata
Data Management Section
Yuya Ikeda, Natuko Iwasaki, Kyouko Hongo, Kaori Tobayama, Tukiko Higuchi, Nami Hirano, Seiko Matsuda, Akiomi Yoshida, Kei Ikeno, Kazuko Tsukamoto. Koichi Asada, Mayumi Yamada, Motoko Nishikawa, Kaoru Atou, Yuuka Sasaki
Information Technology Management Section
Yoshihiro Aoyagi, Rie Terao
- Seeds Development Promotion Division
Seeds Development Support Section
Katshuzi Aikawa, Hisanobu Nomura
Startup Support Section
Nobuyoshi Takeshita
- Clinical Research Coordinating Division
Yasutoshi Kuboki, Masafumi Ikeda, Kiyotaka Yoh, Yoichi Naito, Tomohiro Kadota, Yuichiro Tsukada, Junichiro Yuda, Sumino Kimijima, Ikumi Tamaki, Aya Iwamoto, Kiyoko Adachi, Keiko Yamamoto, Hasumi Okayasu, Yuka Sakamoto, Ayae Minobe, Misato Makiishi, Chiyo Ito, Mariko Ishizuka, Hikari Inagawa, Miyuki Hara, Yuko Ito, Masumi Kudo, Koko Komata, Yoshimi Izumi, Chiharu Hirano, Hiromi Motoyanagi, Rie Taniguchi, Saori Yokoba, Keiko Abe, Yasuko Yoshihara, Yoko Takada, Reiko Sugaya, Hiromi Kawamura, Sayuri Nochi, Naoya Asao, Mayumi Nagino, Tomoko Watanabe, Aki Hashimoto, Ayako Arai, Miwa Chisiki, Yuko Nakanishi, Tamae Yabuki, Hikari Mathuki, Natsuko Takagi, Tomoko Matsumura, Atsuko Katagiri, Yui Ogawa, Noriko Noda,Yoshimi Fujiki, Maki Umezawa, Yukari Hukazawa, Yukiyo Asano, Ai Yamada, Tomoko Suzuki, Mikayo Kutsuna, Kaori Tokushige, Yuki Suzuki, Masahiko Ozaki, Tomoka Okano, Mie Yamada, Kazunori Masai, Mai Hashimoto, Kyoko Uehara, Hiromi Sudo, Satomi Tago, Akiko Yumura, Ayako Kimura, Yuriko Eguchi, Mariko Kamata, Takashi Kojima, Sakie Takasu, Chiaki Shimada, Chikoto Okano, Sanae Wada, Harue Motoki, Yukie Kimura
Introduction
The Clinical Research Support Office supports the clinical trial program conducted in the National Cancer Center Hospital East (NCCHE). The Clinical Research Coordinating Division consists of Clinical Research Coordinating Section, Clinical Trials Administration Section, and Clinical Study Support Section.
The Team and What We Do
- Clinical Research Planning Division
- Clinical Research Management Division
Through the clinical datacenter, study management, site visit monitoring, safety information, and medical writing.
- Seeds Development Promotion Division
Promote seeds development though program management and start-up support.
- Clinical Research Coordinating Division
The Clinical Research Coordinating Division is in charge of supporting clinical research and trials conducted at the hospital, including company- and investigator-initiated clinical trials, post-marketing, and expanded clinical trials, in compliance with the Clinical Research Act and ethical guidelines, etc. The Clinical Trials Administration Section is in charge of administrative procedures, including contracts for clinical trials conducted at the hospital. The Clinical Research Coordinating Section and the Clinical Study Support Section are in charge of subject management (patient interviews, assistance with study explanations, schedule management, observation of adverse events, etc.), data collection management (filling out case report forms), monitoring and auditing, transfer of some research samples, assistance with comprehensive consent explanations to first-time patients at the biobank counter, and support for biobanks that use surplus specimens, etc. In FY2022, the Center supported 499 clinical trials, including 102 newly contracted proposals, for a total of 776 cases (Tables 1, 2, and 3), and obtained consent for biobank blood collection and utilization of surplus specimens from 6,194 patients (consent ratio: 84.4%).
Table 1. Status of Clinical Trials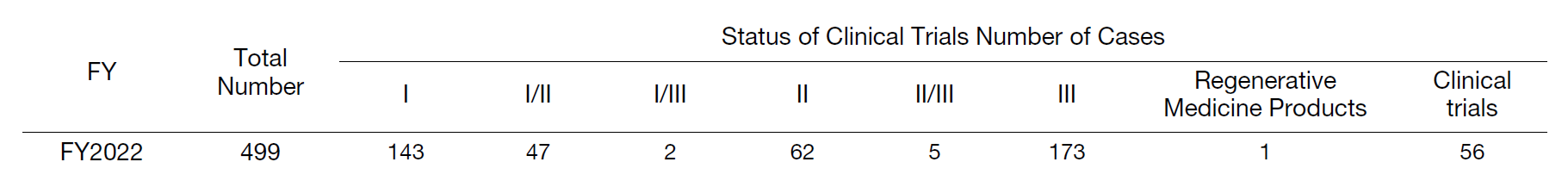

Table 2. Status of New Clinical Trials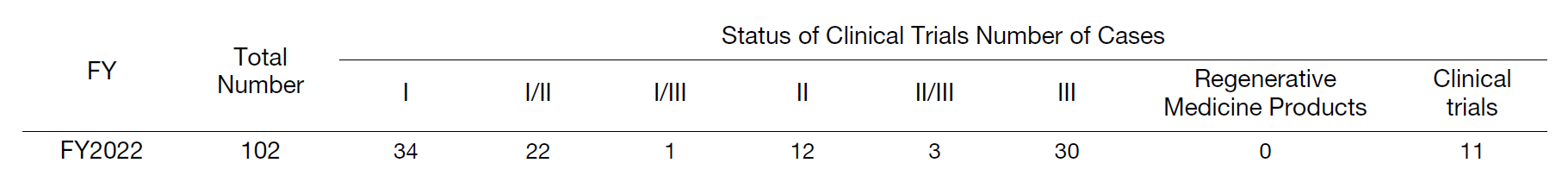


Research Activities
- Started 4 IND trials.
- Supported 7 start-up teams.
- Examined the usefulness of EDC data linkage systems and electronic data in clinical trials.
- Examined the risks in the protocol development stage.
- Performed benchmark-based costing of clinical trials in corporate clinical trials.
- Conducted human resource development for clinical research support, etc.
Education
An educational program for new hires and a training program for rotators are being implemented in accordance with the human resource development rotation. Feedback is provided on the level of proficiency and understanding based on three-monthly evaluations, and a training program for mid-career clinical research coordinators is under construction to help them advance their careers. For CRCs outside the hospital, facility tours and training for advanced-level CRCs were conducted. Regarding work-related risks and problems, cases and corrective actions are shared at regular meetings.
Future Prospects
Major changes have been ongoing in the modalities of anticancer drugs, and the procedures and toxicities to be considered are changing accordingly. To support drug discovery and early development in Japan, we will continue to provide high-quality support while changing the conventional wisdom. At the same time, we will educate and train the next generation of human resources to support this. In addition, as the clinical trial environment has earnestly begun to incorporate digital technology, we will work to create a system that takes patient convenience into consideration while paying attention to safety, and to improve the efficiency of the work of CRCs and other supporting personnel.
