Annual Report 2022
Department of Genetic Medicine and Services
Takeshi Kuwata, Katsuya Tsuchihara, Toru Mukohara, Kiwamu Akagi, Mitsuho Imai, Akiko Nakayama, Sachiyo Mimaki, Yumie Hiraoka, Kaori Kimura, Manami Matsukawa, Chikako Tomozawa, Kenichi Harano, Shingo Matsumoto, Kyoko Toju, Nobuyuki Nakamura, Tsuyoshi Uemoto, Yoshiko Onuma
Introduction
The Department of Genetic Medicine and Services was newly established in May 2016 to handle genetic as well as genomic testing and related issues, including genetic counseling, conducted in the National Cancer Center Hospital East (NCCHE).
The Team and What We Do
The aim of the Department of Genetic Medicine and Services is to deal with the following subjects:
1) Genetic counseling
2) Genetic/genomic testing
3) Ethical issues related to genetic/genomic medicine
4) Educating people on genetic/genomic medicine
5) Regulating the storage and usage of genetic/genomic information
6) Other matters related to genetic/genomic medicine
Accordingly, members of the department with various specialties were assembled, including medical doctors, nurses, genetic counselors and medical technicians from different clinical departments or research laboratories.
To conduct precision oncology, our hospital is designated as a Cancer Genome Medical Core Hospital in Japan and cooperates with 9 affiliated hospitals, especially for providing cancer genome profiling (CGP) tests. Under the national health care services, by March 2023, we performed 296 CGP tests and conducted expert panels for 290 patients, including 374 patients in affiliated hospitals (Figure 1).
The Outpatient Genetic Counseling Clinic provides genetic counseling and genetic testing for cancer patients and their relatives with a familial history and/or specific features suspected for familial cancers. From April 2021 to March 2023, 167 new clients visited the clinic, and a total of 306 genetic counseling sessions were held and 93 genetic testing sessions were provided. Consequently, by March 2023, a total of 669 clients had visited the clinic (Figures 2 and 3). The department also participated in an outpatient clinic specializing in Hereditary Breast and Ovary Cancer (HBOC) syndrome established by the Department of Breast and Medical Oncology.
Figure 1. Number of CGP cases reviewed in expert panels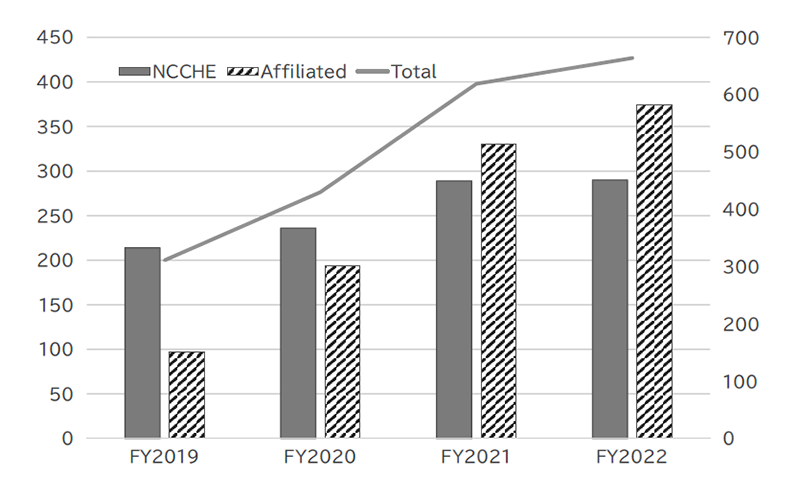

Figure 2. Number of genetic counseling sessions provided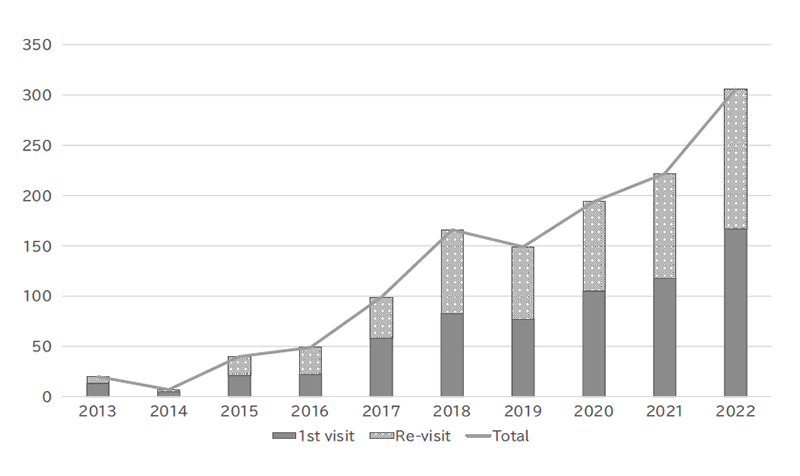

Figure 3. Breakdown of genetic counseling sessions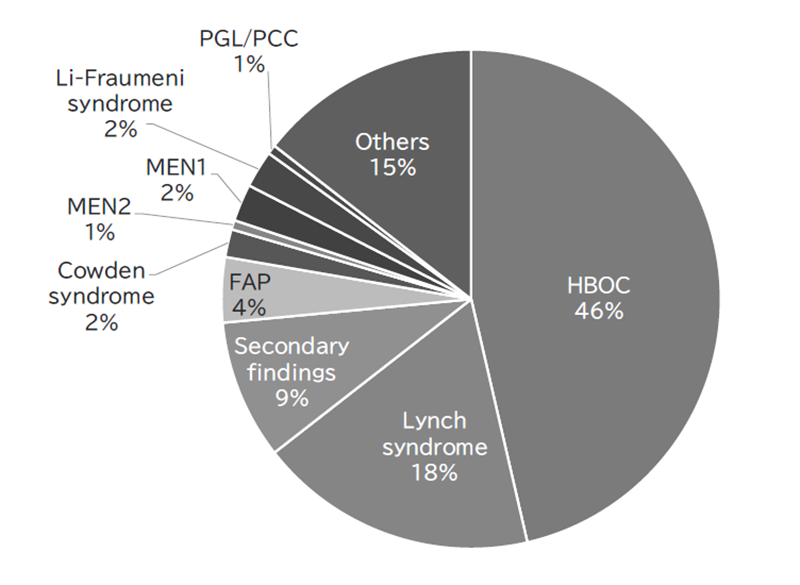

Research Activities
Our Outpatient Genetic Counseling Clinic is participating in the NCC Research and Development Fund program (25-A-1 & 22A-7) and Observational study to evaluate the utility of confirmation of germline mutations in patients with solid tumors and relatives (BRANCH study, UMIN000046085), and provides genetic testing.
Clinical Trials
Our Outpatient Genetic Counseling Clinic supports and provides genetic counseling for patients willing to participate in clinical trials where genetic tests are required.
Education
We educated medical doctors wishing to become board-certified geneticists by attending the Outpatient Genetic Counseling Clinic. We have also provided intramural educational seminars for medical doctors and medical staff to familiarize them with genetic and genomic medicine and provide precision medicine for all cancer patients and their families visiting our hospital. As a Cancer Genome Medical Core Hospital, we also provide seminars and lectures for educating doctors as well as medical staff working at affiliated hospitals.
Future Prospects
We will continue to provide genetic counseling and genetic testing for possible familial cancer patients/families. For precision oncology, we will conduct CGP tests under the national health care system and participate in several clinical trials using new technologies including whole genome/exome sequences. We will also continue to provide education programs for medical doctors and medical staff working in our hospital as well as affiliated hospitals. Our current aim is to establish a genomic testing pipeline from research to clinic to accelerate the development of medical and diagnostic devices for genome medicine.
List of papers published in 2022
Journal
1. Murano T, Ikematsu H, Shinmura K, Okumura K, Kuwata T, Ushiama M, Yoshida T, Takashima K, Nakajo K, Kadota T, Yoda Y, Oono Y, Yano T. Endoscopic management of familial adenomatous polyposis targeting colorectal lesions greater than 5 mm in size: a single-center retrospective study. Familial cancer, 22:83-89, 2023
2. Shimomura A, Yoshida M, Kubo T, Yamashita S, Noguchi E, Nagayama A, Hanamura T, Okazaki M, Mukohara T, Tsuruga A, Tanaka K, Kawamura Y, Higuchi T, Takahashi Y, Kurozumi S, Hayashida T, Ichikawa H, Ushijima T, Suto A. Clinicopathological features, genetic alterations, and BRCA1 promoter methylation in Japanese male patients with breast cancer. Breast cancer research and treatment, 197:593-602, 2023
3. Morii E, Hatanaka Y, Motoi N, Kawahara A, Hamakawa S, Kuwata T, Nagatomo T, Oda Y, Okamoto A, Tanaka R, Iyoda A, Ichiro M, Matsuo Y, Nakamura N, Nakai T, Fukuhara M, Tokita K, Yamaguchi T, Takenaka M, Kawabata A, Hatanaka KC, Tsubame K, Satoh Y. Guidelines for Handling of Cytological Specimens in Cancer Genomic Medicine. Pathobiology, 1-23, 2023
4. Tamura K, Mukohara T, Yonemori K, Kawabata Y, Nicolas X, Tanaka T, Iwata H. Phase 1 study of oral selective estrogen receptor degrader (SERD) amcenestrant (SAR439859), in Japanese women with ER-positive and HER2-negative advanced breast cancer (AMEERA-2). Breast cancer (Tokyo, Japan), 30:506-517, 2023
5. Funasaka C, Naito Y, Kusuhara S, Nakao T, Nakajima H, Kawamoto M, Baba K, Mamishin K, Kondoh C, Harano K, Matsubara N, Hosono A, Sasaki T, Kawasaki T, Mukohara T. Clinical features of CDK4/6 inhibitor-related interstitial lung disease in patients with breast cancer: a case series study. Japanese journal of clinical oncology, 53:105-114, 2023
6. Koganemaru S, Kawai T, Fuchigami H, Maeda N, Koyama K, Kuboki Y, Mukohara T, Doi T, Yasunaga M. Quantitative analysis of drug distribution in heterogeneous tissues using dual-stacking capillary electrophoresis-mass spectrometry. British journal of pharmacology, 180:762-774, 2023
7. Akimoto E, Kuwata T, Shitara K, Kawazoe A, Sakamoto N, Ishii G, Ochiai A, Kinoshita T. Impact of Programmed Death-Ligand 1 Expression on Mismatch Repair Deficiency and Epstein-Barr Virus Status on Survival Outcomes in Patients with Stage II/III Gastric Cancer After Surgery. Annals of surgical oncology, 2023
8. Kubota Y, Kawazoe A, Mishima S, Nakamura Y, Kotani D, Kuboki Y, Bando H, Kojima T, Doi T, Yoshino T, Kuwata T, Shitara K. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO open, 8:100762, 2023
9. Nakai C, Mimaki S, Matsushima K, Shinozaki E, Yamazaki K, Muro K, Yamaguchi K, Nishina T, Yuki S, Shitara K, Bando H, Suzuki Y, Akagi K, Nomura S, Fujii S, Sugiyama M, Nishida N, Mizokami M, Koh Y, Koshizaka T, Okada H, Abe Y, Ohtsu A, Yoshino T, Tsuchihara K. Regulation of MEK inhibitor selumetinib sensitivity by AKT phosphorylation in the novel BRAF L525R mutant. International journal of clinical oncology, 28:654-663, 2023
10. Nishio S, Yonemori K, Usami T, Minobe S, Yunokawa M, Iwata T, Okamoto A, Aoki Y, Itamochi H, Takekuma M, Harano K, Yamamoto K, Maruko T, Ugai H, Tekin C, Colombo N, Fujiwara K, Hasegawa K, Ushijima K. Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: Results from KEYNOTE-826. Cancer science, 113:3877-3887, 2022
11. Masuda N, Ono M, Mukohara T, Yasojima H, Shimoi T, Kobayashi K, Harano K, Mizutani M, Tanioka M, Takahashi S, Kogawa T, Suzuki T, Okumura S, Takase T, Nagai R, Semba T, Zhao ZM, Ren M, Yonemori K. Phase 1 study of the liposomal formulation of eribulin (E7389-LF): Results from the breast cancer expansion cohort. European journal of cancer (Oxford, England : 1990), 168:108-118, 2022
12. Mizuno M, Chiba I, Mukohara T, Kondo M, Maruo K, Ohigashi T, Naruo M, Asano Y, Onishi T, Tanabe H, Muta R, Mishima S, Okano S, Yuda M, Hosono A, Ueda Y, Bando H, Itagaki H, Ferrans CE, Akimoto T. Effectiveness of an online support program to help female cancer patients manage their health and illness: Protocol for a randomized controlled trial. Contemporary clinical trials communications, 30:101035, 2022
13. Eguchi Y, Nakai T, Kojima M, Wakabayashi M, Sakamoto N, Sakashita S, Miyazaki S, Taki T, Watanabe R, Watanuki R, Yamauchi C, Iwatani T, Mukohara T, Onishi T, Ishii G. Pathologic method for extracting good prognosis group in triple-negative breast cancer after neoadjuvant chemotherapy. Cancer science, 113:1507-1518, 2022
14. Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, Kawabata H, Masuda N, Futamura M, Minami H, Matsumoto K, Yoshimura K, Kitano S, Takano T. Safety and efficacy of nivolumab plus bevacizumab, paclitaxel for HER2-negative metastatic breast cancer: Primary results and biomarker data from a phase 2 trial (WJOG9917B). European journal of cancer (Oxford, England : 1990), 171:193-202, 2022
15. Niguma K, Mamishin K, Naito Y, Nomura S, Wakabayashi M, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Onishi T, Kawasaki T, Mukohara T. Impact of Older Age and Medico-social Factors on the Decision to Offer Adjuvant Chemotherapy to Patients With Breast Cancer. Anticancer research, 42:3743-3751, 2022
16. Ozaki Y, Tsurutani J, Mukohara T, Iwasa T, Takahashi M, Tanabe Y, Kawabata H, Masuda N, Futamura M, Minami H, Matsumoto K, Yoshimura K, Kitano S, Takano T. Data of programmed death-ligand 1 expression and VEGF: Nivolumab, bevacizumab and paclitaxel For HER2-negative metastatic breast cancer. Data in brief, 45:108558, 2022
17. Shirai K, Guan G, Meihui T, Xiaoling P, Oka Y, Takahashi Y, Bhagat AAS, Yanagida M, Iwanaga S, Matsubara N, Mukohara T, Yoshida T. Hybrid double-spiral microfluidic chip for RBC-lysis-free enrichment of rare cells from whole blood. Lab on a chip, 22:4418-4429, 2022
18. Kuboki Y, Shimizu T, Yonemori K, Kojima T, Kondo S, Koganemaru S, Iwasa S, Harano K, Koyama T, Lu V, Zhou X, Niu H, Yanai T, Garcia-Ribas I, Doi T, Yamamoto N. Safety, Tolerability, and Pharmacokinetics of TAK-931, a Cell Division Cycle 7 Inhibitor, in Patients with Advanced Solid Tumors: A Phase I First-in-Human Study. Cancer research communications, 2:1426-1435, 2022
19. Tamiya Y, Nakai T, Suzuki A, Mimaki S, Tsuchihara K, Sato K, Yoh K, Matsumoto S, Zenke Y, Nosaki K, Izumi H, Shibata Y, Sakai T, Taki T, Miyazaki S, Watanabe R, Sakamoto N, Sakashita S, Kojima M, Hashimoto N, Tsuboi M, Goto K, Ishii G. The impact of tertiary lymphoid structures on clinicopathological, genetic and gene expression characteristics in lung adenocarcinoma. Lung cancer (Amsterdam, Netherlands), 174:125-132, 2022
20. Chida K, Kawazoe A, Suzuki T, Kawazu M, Ueno T, Takenouchi K, Nakamura Y, Kuboki Y, Kotani D, Kojima T, Bando H, Mishima S, Kuwata T, Sakamoto N, Watanabe J, Mano H, Ikeda M, Shitara K, Endo I, Nakatsura T, Yoshino T. Transcriptomic Profiling of MSI-H/dMMR Gastrointestinal Tumors to Identify Determinants of Responsiveness to Anti-PD-1 Therapy. Clinical cancer research, 28:2110-2117, 2022
21. Yukami H, Kawazoe A, Lin YT, Koyama S, Fukuoka S, Hara H, Takahashi N, Kojima T, Asayama M, Yoshii T, Bando H, Kotani D, Nakamura Y, Kuboki Y, Mishima S, Wakabayashi M, Kuwata T, Goto M, Higuchi K, Yoshino T, Doi T, Nishikawa H, Shitara K. Updated Efficacy Outcomes of Anti-PD-1 Antibodies plus Multikinase Inhibitors for Patients with Advanced Gastric Cancer with or without Liver Metastases in Clinical Trials. Clinical cancer research, 28:3480-3488, 2022
22. Aoki Y, Kawazoe A, Kubota Y, Chida K, Mishima S, Kotani D, Nakamura Y, Kuboki Y, Bando H, Kojima T, Doi T, Yoshino T, Kuwata T, Shitara K. Characteristics and clinical outcomes of patients with advanced gastric or gastroesophageal cancer treated in and out of randomized clinical trials of first-line immune checkpoint inhibitors. International journal of clinical oncology, 27:1413-1420, 2022
23. Morinaga T, Inozume T, Kawazu M, Ueda Y, Sax N, Yamashita K, Kawashima S, Nagasaki J, Ueno T, Lin J, Ohara Y, Kuwata T, Yukami H, Kawazoe A, Shitara K, Honobe-Tabuchi A, Ohnuma T, Kawamura T, Umeda Y, Kawahara Y, Nakamura Y, Kiniwa Y, Morita A, Ichihara E, Kiura K, Enokida T, Tahara M, Hasegawa Y, Mano H, Suzuki Y, Nishikawa H, Togashi Y. Mixed Response to Cancer Immunotherapy is Driven by Intratumor Heterogeneity and Differential Interlesion Immune Infiltration. Cancer research communications, 2:739-753, 2022
24. Tanaka S, Umemoto K, Kubo S, Sato Y, Mimaki S, Tsuchihara K, Takemura S, Shinkawa H, Mori A, Ikeda M. Nivolumab for treating patients with occupational cholangiocarcinoma. Journal of hepato-biliary-pancreatic sciences, 29:1153-1155, 2022
25. Yanagihara K, Iino Y, Yokozaki H, Kubo T, Oda T, Kubo T, Komatsu M, Sasaki H, Ichikawa H, Kuwata T, Seyama T, Ochiai A. A Comparative Study of Patient-Derived Tumor Models of Pancreatic Ductal Adenocarcinoma Involving Orthotopic Implantation. Pathobiology, 89:222-232, 2022
26. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, Kamada T, Irie T, Okumura G, Kono H, Ito D, Fujii R, Watanabe S, Sai A, Fukuoka S, Sugiyama E, Watanabe G, Owari T, Nishinakamura H, Sugiyama D, Maeda Y, Kawazoe A, Yukami H, Chida K, Ohara Y, Yoshida T, Shinno Y, Takeyasu Y, Shirasawa M, Nakama K, Aokage K, Suzuki J, Ishii G, Kuwata T, Sakamoto N, Kawazu M, Ueno T, Mori T, Yamazaki N, Tsuboi M, Yatabe Y, Kinoshita T, Doi T, Shitara K, Mano H, Nishikawa H. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer cell, 40:201-218.e9, 2022
