Annual Report 2022
Department of Clinical Laboratories
Hiroshi Yamakawa, Masahiro Karibe, Narumi Akimoto, Yuko Adegawa, Momoko Iguchi, Kenta Akie, Michiteru Yamagishi, Atsuko Uno, Masaki Takeda, Takuya Aiba, Mika Narikiyo, Nobuyuki Nakamura, Go Sato, Masayuki Ito, Riko Kawarai, Kazuma Takada, Yuki Takano, Masako Naito, Mari Hisano, Mitsunori Tajima, Yui Jinbo, Tomofumi Tan, Saki Nakamura, Akira Miyaura, Keisei Nakamura, Kimihiko Kawamura, Yasuharu Hashimoto, Mika Sasanuma, Takuya Hanazawa, Momoka Sakayori, Haruka Nozaki, Ayaka Takahashi, Daiki Ito, Madoka Nakayama, Mana Shimamura, Fubuki Omoya, Keiko Nakai, Ryota Utsumi, Atsuko Suzuki, Kozue Saito, Hitomi Chikuta, Momoko Murata, Nodoka Tanago, Sayuri Shibayama, Tomomi Wachi, Masayuki Sukegawa, Takaki Kobayashi, Katsuhiro Sato, Kentaro Yamada, Yuichiro Yazaki, Shota Oishi, Yuma Furuya, Hikari Sekiguchi, Jun Ikoma, Masahiro Inoue, Tomohiro Sakuma, Aiko Kimura, Ikumi Komatsuda, Keiko Katsuno, Yumi Takeuchi, Ayumi Iwaya, Aya Koike, Tomoko Ikeda, Tomoe Katakura, Kaori Toyama, Ayaka Ohashi, Yoriko Furusawa, Michiko Iida, Izumi Suzuki, Sachiko Fukuda, Megumi Yamaguchi, Mariko Kinoshita, Chiho Furuya, Noriko Sato, Kyoko Kuwayama, Kanako Nakayama
Introduction
In order to promote the best cancer treatment, which is our hospital's philosophy, the Department of Clinical Laboratory is committed to "providing the best cancer treatment,'' "research, development and dissemination of new medical care,'' and "supporting Japan's efforts through education and training.'' We carry out activities to improve medical care and disseminate information domestically and internationally. We provide the best clinical tests for patients at the Hospital East through three pillars: medical support, research, and education.
The Team and What We Do
The total number of clinical examinations was 4,552,503, an increase of about 5% from the previous year, and it is increasing year by year. (Table 1). In particular, with the opening of the Department of Cardiovascular Medicine in April 2022, the number of cardiac ultrasounds increased by 105% compared to the previous year, and the number of lower extremity ultrasounds increased by 146%.
Compared to the previous year, the number of clinical trial tests was 94% for specimen processing (PK), 105% for electrocardiography, 73% for fundus examination, and 96% for pathological specimen preparation (Table 2).
In August 2022, we obtained CAP accreditation (College of American Pathologists) (Figure 1).
CAP accreditation enables us to statistically compare our own clinical laboratory data with the data from laboratories around the world, and to provide more accurate laboratory data by recognizing internationally mainstream testing methods.
Table 1. Number of clinical tests performed in FY2022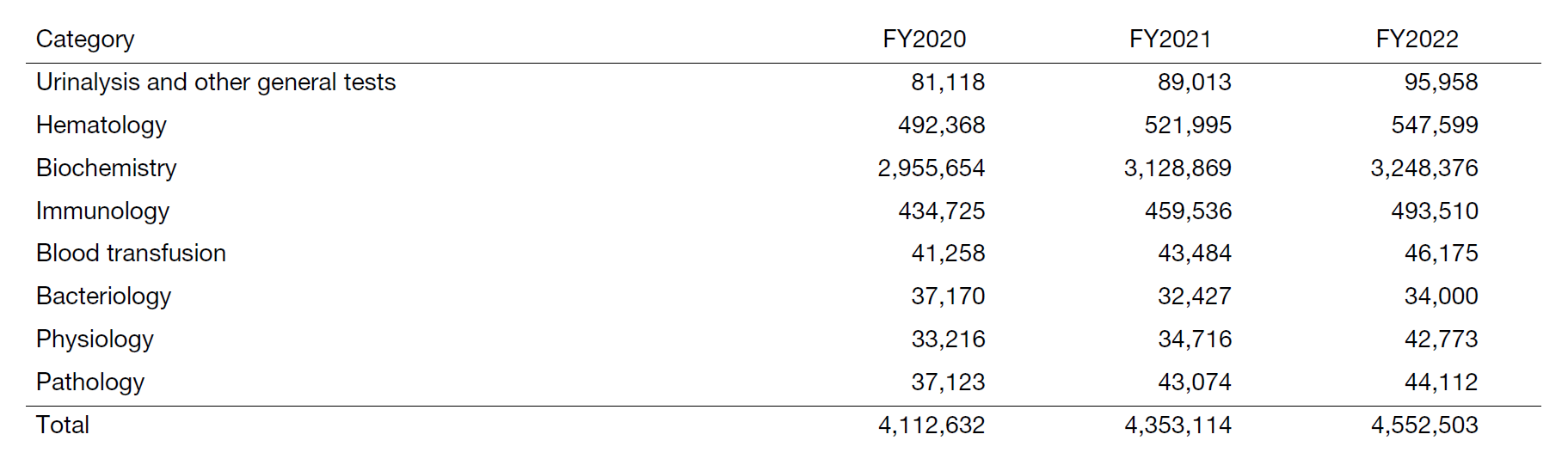

Table 2. Number of clinical trial tests performed in FY2022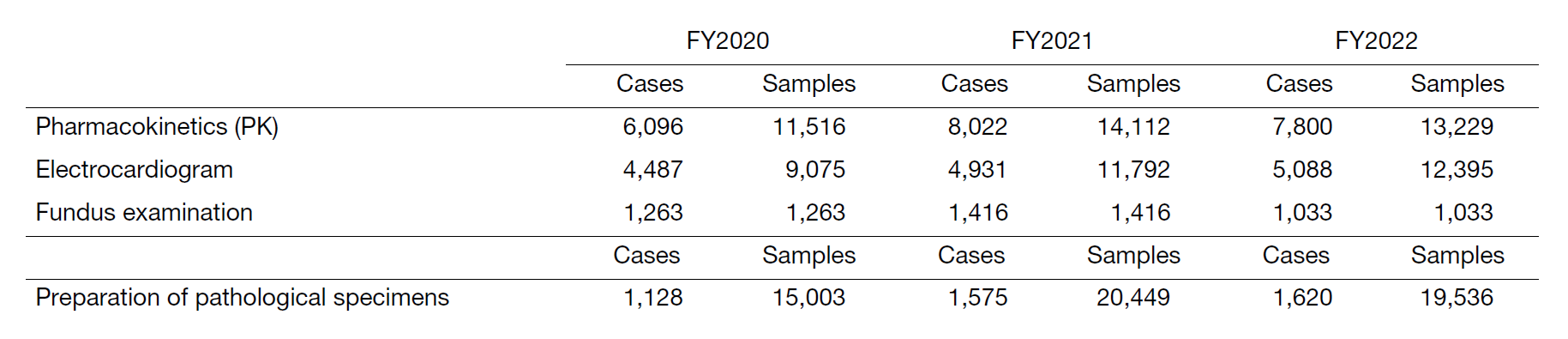


Research Activities
- Fixed accuracy control range in automatic blood cell analyzer
Statistical processing was performed using the standard deviation of past lots as a population, and the control range of the automatic blood cell analyzer was fixed. As a result, the data collection period, which previously required 10 days, was shortened to 5 days, and the number of control sample measurements per day was reduced from 3.4 to 2.2.
- Introduction of LBC (liquid-based cytology) for respiratory specimens
In FY2022, 164 LBCs (127 additional searches using immunostaining) were conducted.
When there are a small number of atypical cells, it is now possible to prepare an LBC specimen to prevent false negatives and avoid ambiguous judgments.
Education
Study sessions, training sessions, lectures, etc. were held once a month to improve the academic knowledge, skills, and ethics of our staff.
Regarding qualifications, we had one certified pathological laboratory technician, one certified general laboratory technician, two ultrasonographers, and five persons who passed various second-level examinations.
From October 2022, one member has been job-rotated to the Department of Medical Information with the aim of improving the hospital's functions by allowing the employees to experience duties in other areas within the hospital
Two facilities accepted five clinical trainees.
Future Prospects
As a clinical laboratory facility that has obtained ISO15189 certification and CAP accreditation (College of American Pathologists), which are international standards, we conduct medical treatment and clinical research in the Hospital East. In the future, we will strengthen collaboration with other departments of the Hospital East as well as the Exploratory Oncology Research & Clinical Trial Center, and actively participate in the development of new tests and equipment. We will contribute to the implementation of Hospital East's vision of "providing the world's best cancer care and creating new, world-class cancer care.''
List of papers published in 2022
Journal
1. Morii E, Hatanaka Y, Motoi N, Kawahara A, Hamakawa S, Kuwata T, Nagatomo T, Oda Y, Okamoto A, Tanaka R, Iyoda A, Ichiro M, Matsuo Y, Nakamura N, Nakai T, Fukuhara M, Tokita K, Yamaguchi T, Takenaka M, Kawabata A, Hatanaka KC, Tsubame K, Satoh Y. Guidelines for Handling of Cytological Specimens in Cancer Genomic Medicine. Pathobiology, 1-23, 2023
