Annual Report 2022
Department of Head and Neck, Esophageal Medical Oncology
Ken Kato, Yoshitaka Honma, Shun Yamamoto, Kazuki Yokoyama, Akihiro Ohara, Mai Itoyama
Introduction
The Department of Head and Neck, Esophageal Medical Oncology focuses on the development of new drugs and establishment of standard chemotherapy regimens, including multimodality treatment with surgery and/or radiotherapy for advanced head and neck cancers (HNCs), consisting of malignancies arising from the oral cavity, nasopharynx, oropharynx, hypopharynx, larynx, nasal/paranasal cavity, salivary gland, ear canal, and thyroid, etc. We also focus on the multimodality treatment of esophageal cancer (EC), mainly chemotherapy and chemoradiotherapy. The main histology of HNC and EC is squamous cell carcinoma. However, there is still a wide variety of histological types, especially in the nasal/paranasal cavity, salivary glands, and gastroesophageal-junctional cancer. Therefore, the pathological diagnosis is essential, making a treatment strategy based on pathological findings significant in advanced HNCs and ECs.
The Team and What We Do
In the fiscal year 2022, we treated a total of 632 inpatients, of which 399 were newly diagnosed cases. The breakdown included 216 patients with esophageal cancer/esophagogastric junction cancer, 8 with esophageal neuroendocrine tumors, 20 with hypopharyngeal cancer, 28 with oropharyngeal cancer, 26 with oral cavity cancer, 13 with laryngeal cancer, 8 with nasopharyngeal cancer, and 80 with other rare head and neck cancers. We have also received referrals from other institutions for rare head and neck cancers, such as undifferentiated thyroid cancer and salivary gland cancer.
Research Activities
We have conducted numerous corporate, physician-initiated, and multi-center clinical trials. As the lead author, we have presented at scientific conferences and authored papers. In the fiscal year 2022, a total of 102 patients were registered for clinical trials/studies (Table 1). We presented a total of 76 conference presentations (including co-authorships), 34 of which were presented at international conferences. We also published 54 papers (including co-authored ones), 42 of which were original articles in English and 7 were English reviews. Among these, our department served as the lead or corresponding author in 15 papers. We maintain a list of clinical trials/studies conducted by our department, along with the number of registrations (Table 1).
Table 1. Clinical trials and the number of registered patients.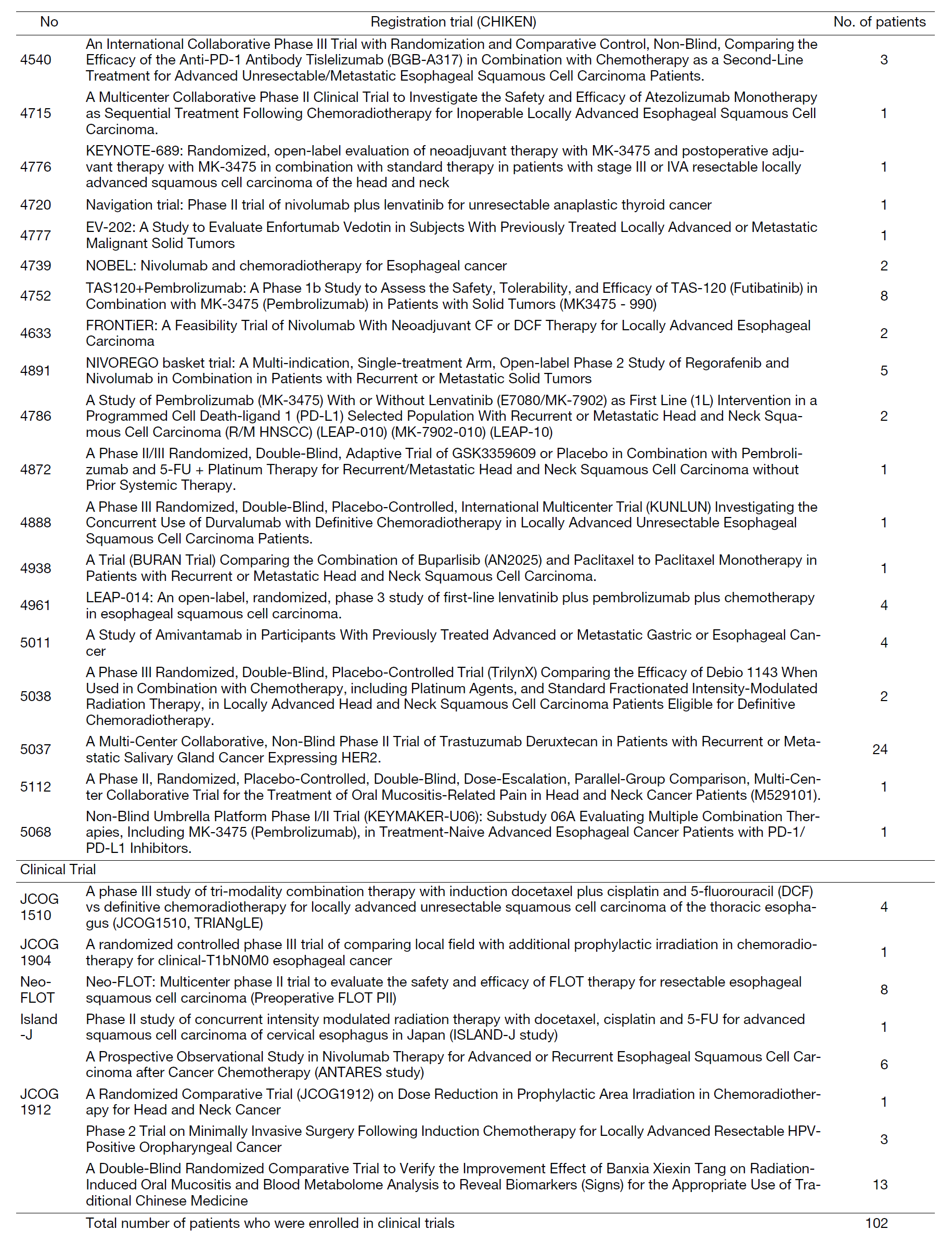

Clinical Trials
With the aim of establishing effective treatments, we are actively conducting clinical research. Our staff plays a central role in proposing and participating in new trials initiated by the Japan Clinical Oncology Group (JCOG), and our institution actively registers patients, contributing to the establishment of new standard treatments. We not only engage in late-stage treatment development but also participate in domestic and international clinical trials from the planning stage to phases I-III for drug development. In addition, we coordinate clinical trials led by physicians for the preoperative treatment of esophageal cancer and register numerous cases as a participating institution for physician-led trials on salivary gland cancer. We are actively involved in translational research (TR) in collaboration with research institutes and other facilities, proposing new physician-led trials to pharmaceutical companies.
Education
As Head and Neck, Esophageal Medicine residents, Dr. Mai Itoyama and Dr. Akihiro Ohara, who are originally from other university otolaryngology departments, have been studying comprehensive treatments for head and neck cancer and esophageal cancer at our institution, expanding the entry of non-internal medicine physicians into the field of medical oncology. We conduct daily rounds and provide education to residents and staff members through conferences. Each staff member provides lectures to residents every three months, and we also distribute them as Zoom or video archives to institutions with former residents and related facilities. We regularly conduct exchange activities with institutions such as Juntendo University Department of Gastroenterology and Nagasaki University Otolaryngology, using Zoom for case conferences and lectures. We are actively attempting to discover new talents. Resident-led presentations at conferences included 18 presentations (9 domestic and 9 international) and 11 English articles.
Future Prospects
The treatment of head and neck cancer and esophageal cancer has not yet reached many regions, therefore we collaborate with the Regional Medical Coordination Office to increase referrals to our hospital. While we base our treatments on standard practices, we actively strive for continuous improvement to meet the unmet needs of patients, aiming to establish a brand that makes other hospitals want to refer patients to us. On the research front, we actively engage with global teams from pharmaceutical companies at conferences and propose research ideas, highlighting the strengths of our hospital, which boasts one of the world's largest patient populations. We will continue to propose physician-initiated trials and conduct multinational clinical trials in Asia. Collaborating with the research institute and our hospital's Clinical Development Promotion Division, we will focus on translational research (TR) to develop new treatments. In terms of education, through resident education, we aim to train not only highly specialized professionals in cancer drug therapy but also young oncologists with research-oriented minds who are well-versed in comprehensive treatments, including surgery and radiation therapy. We aspire to nurture the next generation of researchers.
List of papers published in 2022
Journal
1. Omura G, Honma Y, Matsumoto Y, Shinozaki T, Itoyama M, Eguchi K, Sakai T, Yokoyama K, Watanabe T, Ohara A, Kato K, Yoshimoto S. Transnasal photoimmunotherapy with cetuximab sarotalocan sodium: Outcomes on the local recurrence of nasopharyngeal squamous cell carcinoma. Auris, nasus, larynx, 50:641-645, 2023
2. Matsuzaki J, Kato K, Oono K, Tsuchiya N, Sudo K, Shimomura A, Tamura K, Shiino S, Kinoshita T, Daiko H, Wada T, Katai H, Ochiai H, Kanemitsu Y, Takamaru H, Abe S, Saito Y, Boku N, Kondo S, Ueno H, Okusaka T, Shimada K, Ohe Y, Asakura K, Yoshida Y, Watanabe SI, Asano N, Kawai A, Ohno M, Narita Y, Ishikawa M, Kato T, Fujimoto H, Niida S, Sakamoto H, Takizawa S, Akiba T, Okanohara D, Shiraishi K, Kohno T, Takeshita F, Nakagama H, Ota N, Ochiya T. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI cancer spectrum, 7:pkac080, 2023
3. Kadono T, Yamamoto S, Hirose T, Ikeda G, Ohara A, Itoyama M, Yokoyama K, Honma Y, Hashimoto T, Sekine S, Ishiyama K, Oguma J, Daiko H, Kato K. Safety and short-term efficacy of preoperative FOLFOX therapy in patients with resectable esophageal squamous cell carcinoma who are ineligible for cisplatin. Esophagus, 20:109-115, 2023
4. Kato K, Doki Y, Ogata T, Motoyama S, Kawakami H, Ueno M, Kojima T, Shirakawa Y, Okada M, Ishihara R, Kubota Y, Amaya-Chanaga C, Chen T, Matsumura Y, Kitagawa Y. First-line nivolumab plus ipilimumab or chemotherapy versus chemotherapy alone in advanced esophageal squamous cell carcinoma: a Japanese subgroup analysis of open-label, phase 3 trial (CheckMate 648/ONO-4538-50). Esophagus, 20:291-301, 2023
5. Mitani S, Kato K, Daiko H, Ito Y, Nozaki I, Kojima T, Yano M, Nakagawa S, Ueno M, Watanabe M, Tsunoda S, Abe T, Kadowaki S, Kadota T, Sasaki K, Machida R, Kitagawa Y. Second primary malignancies in patients with clinical T1bN0 esophageal squamous cell carcinoma after definitive therapies: supplementary analysis of the JCOG trial: JCOG0502. Journal of gastroenterology, 57:455-463, 2022
6. Takeuchi H, Ito Y, Machida R, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Okuno T, Hironaka S, Nozaki I, Ura T, Chin K, Kojima T, Seki S, Sakanaka K, Fukuda H, Kitagawa Y. A Single-Arm Confirmatory Study of Definitive Chemoradiation Therapy Including Salvage Treatment for Clinical Stage II/III Esophageal Squamous Cell Carcinoma (JCOG0909 Study). International journal of radiation oncology, biology, physics, 114:454-462, 2022
7. Takemura C, Kashima J, Hashimoto T, Ichikawa H, Honma Y, Goto Y, Watanabe SI, Yatabe Y. A mimic of lung adenocarcinoma: a case report of histological conversion of metastatic thyroid papillary carcinoma. Histopathology, 80:1004-1007, 2022
8. Ida H, Koyama T, Mizuno T, Sunami K, Kubo T, Sudo K, Tao K, Hirata M, Yonemori K, Kato K, Okusaka T, Ohe Y, Matsui Y, Yamazaki N, Ogawa C, Kawai A, Narita Y, Esaki M, Yamamoto N. Clinical utility of comprehensive genomic profiling tests for advanced or metastatic solid tumor in clinical practice. Cancer science, 113:4300-4310, 2022
9. Nozaki I, Machida R, Kato K, Daiko H, Ito Y, Kojima T, Yano M, Ueno M, Nakagawa S, Kitagawa Y. Long-term survival of patients with T1bN0M0 esophageal cancer after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter trial. Surgical endoscopy, 36:4275-4282, 2022
10. Oguma J, Ishiyama K, Kurita D, Kanematsu K, Fujii Y, Kubo K, Yamamoto S, Honma Y, Kato K, Daiko H. Novel pathological staging for patients with locally advanced esophageal squamous cell carcinoma undergoing neoadjuvant chemotherapy followed by surgery. Esophagus, 19:214-223, 2022
11. Suzuki K, Yamaguchi T, Kohda M, Tanaka M, Takemura H, Wakita M, Tabe Y, Kato S, Nasu M, Hashimoto T, Mine S, Serizawa N, Tomishima K, Nagahara A, Matsuda T, Yamaji T, Tsugane S, Saito Y, Daiko H, Yoshikawa T, Kato K, Okusaka T, Ochiya T, Yamamoto Y, Yotsui S, Yamamoto T, Yamasaki T, Miyata H, Yasui M, Omori T, Ohkawa K, Ikezawa K, Nakabori T, Sugimoto N, Kudo T, Yoshida K, Ohue M, Nishizawa T. Establishment of preanalytical conditions for microRNA profile analysis of clinical plasma samples. PloS one, 17:e0278927, 2022
12. Tsukamoto S, Honma Y, Shoji H, Hirano H, Inoue M, Takamizawa Y, Moritani K, Imaizumi J, Kanemitsu Y. Clinical outcomes of surgical and imatinib treatment for rectal gastrointestinal stromal tumours: retrospective cohort study. BJS open, 6:zrac067, 2022
13. Shibayama T, Shimoi T, Mori T, Noguchi E, Honma Y, Hijioka S, Yoshida M, Ogawa C, Yonemori K, Yatabe Y, Yoshida A. Cytokeratin-positive Malignant Tumor in the Abdomen With EWSR1/FUS-CREB Fusion: A Clinicopathologic Study of 8 Cases. The American journal of surgical pathology, 46:134-146, 2022
14. Kang YK, Morita S, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Sameshima H, Chen LT, Boku N. Exploration of predictors of benefit from nivolumab monotherapy for patients with pretreated advanced gastric and gastroesophageal junction cancer: post hoc subanalysis from the ATTRACTION-2 study. Gastric cancer, 25:207-217, 2022
15. Sakai M, Kitagawa Y, Saeki H, Miyazaki T, Yamaji T, Nemoto K, Oyama T, Muto M, Takeuchi H, Toh Y, Matsubara H, Mano M, Kono K, Kato K, Yoshida M, Kawakubo H, Booka E, Yamatsuji T, Kato H, Ito Y, Ishikawa H, Ishihara R, Tsushima T, Kawachi H, Oyama T, Kojima T, Kuribayashi S, Makino T, Matsuda S, Sohda M, Kubo Y, Doki Y. Fruit and vegetable consumption and risk of esophageal cancer in the Asian region: a systematic review and meta-analysis. Esophagus, 19:27-38, 2022
16. Muro K, Kojima T, Moriwaki T, Kato K, Nagashima F, Kawakami H, Ishihara R, Ogata T, Satoh T, Iwakami K, Han S, Yatsuzuka N, Takami T, Bhagia P, Doi T. Second-line pembrolizumab versus chemotherapy in Japanese patients with advanced esophageal cancer: subgroup analysis from KEYNOTE-181. Esophagus, 19:137-145, 2022
17. Kubo Y, Kitagawa Y, Miyazaki T, Sohda M, Yamaji T, Sakai M, Saeki H, Nemoto K, Oyama T, Muto M, Takeuchi H, Toh Y, Matsubara H, Mano M, Kono K, Kato K, Yoshida M, Kawakubo H, Booka E, Yamatsuji T, Kato H, Ito Y, Ishikawa H, Ishihara R, Tsushima T, Kawachi H, Oyama T, Kojima T, Kuribayashi S, Makino T, Matsuda S, Doki Y. The potential for reducing alcohol consumption to prevent esophageal cancer morbidity in Asian heavy drinkers: a systematic review and meta-analysis. Esophagus, 19:39-46, 2022
18. Oshima K, Kato K, Ito Y, Daiko H, Nozaki I, Nakagawa S, Shibuya Y, Kojima T, Toh Y, Okada M, Hironaka S, Akiyama Y, Komatsu Y, Maejima K, Nakagawa H, Onuki R, Nagai M, Kato M, Kanato K, Kuchiba A, Nakamura K, Kitagawa Y. Prognostic biomarker study in patients with clinical stage I esophageal squamous cell carcinoma: JCOG0502-A1. Cancer science, 113:1018-1027, 2022
19. Kashihara T, Ishiki H, Kato K. Definitive Chemoradiotherapy for Older Patients With Esophageal Cancer. JAMA oncology, 8:304-305, 2022
20. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. The Lancet. Oncology, 23:234-247, 2022
21. Mori Y, Kikuchi O, Horimatsu T, Hara H, Hironaka S, Kojima T, Kato K, Tsushima T, Ishihara R, Mukai K, Uozumi R, Tada H, Kasai H, Kawaguchi A, Muto M. Multicenter phase II study of trifluridine/tipiracil for esophageal squamous carcinoma refractory/intolerant to 5-fluorouracil, platinum compounds, and taxanes: the ECTAS study. Esophagus, 19:444-451, 2022
22. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. The New England journal of medicine, 386:449-462, 2022
23. Kato K, Kadota T, Abe S. Reply. Gastroenterology, 162:2130-2131, 2022
24. Nakamura Y, Okamoto W, Denda T, Nishina T, Komatsu Y, Yuki S, Yasui H, Esaki T, Sunakawa Y, Ueno M, Shinozaki E, Matsuhashi N, Ohta T, Kato K, Ohtsubo K, Bando H, Hara H, Satoh T, Yamazaki K, Yamamoto Y, Okano N, Terazawa T, Kato T, Oki E, Tsuji A, Horita Y, Hamamoto Y, Kawazoe A, Nakajima H, Nomura S, Mitani R, Yuasa M, Akagi K, Yoshino T. Clinical Validity of Plasma-Based Genotyping for Microsatellite Instability Assessment in Advanced GI Cancers: SCRUM-Japan GOZILA Substudy. JCO precision oncology, 6:e2100383, 2022
25. Suzuki K, Igata H, Abe M, Yamamoto Y. Multiple cancer type classification by small RNA expression profiles with plasma samples from multiple facilities. Cancer science, 113:2144-2166, 2022
26. Tsubokura M, Adegawa Y, Kojima M, Tanosaki R, Ohtake R, Kase Y, Iwashita N, Kasane M, Nakabayashi S, Takeuchi S, Kato K, Boku N, Kanemitsu Y, Okusaka T, Fujimoto H, Yonemori K, Ishiki H, Kawamura K, Satomi E, Matsushita H. Adverse effects of cell-free and concentrated ascites reinfusion therapy for malignant ascites: a single-institute experience. BMC cancer, 22:268, 2022
27. Okada M, Kato K, Cho BC, Takahashi M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Matsumura Y, Takazawa A, Kitagawa Y. Three-Year Follow-Up and Response-Survival Relationship of Nivolumab in Previously Treated Patients with Advanced Esophageal Squamous Cell Carcinoma (ATTRACTION-3). Clinical cancer research, 28:3277-3286, 2022
28. Inoue T, Ishihara R, Shibata T, Suzuki K, Kitagawa Y, Miyazaki T, Yamaji T, Nemoto K, Oyama T, Muto M, Takeuchi H, Toh Y, Matsubara H, Mano M, Kono K, Kato K, Yoshida M, Kawakubo H, Booka E, Yamatsuji T, Kato H, Ito Y, Ishikawa H, Tsushima T, Kawachi H, Oyama T, Kojima T, Kuribayashi S, Makino T, Matsuda S, Doki Y. Endoscopic imaging modalities for diagnosing the invasion depth of superficial esophageal squamous cell carcinoma: a systematic review. Esophagus, 19:375-383, 2022
29. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, Yu X, Shu Y, Luo Q, Wang J, Chen Z, Niu Z, Zhang L, Yi T, Sun JM, Chen J, Yu G, Lin CY, Hara H, Bi Q, Satoh T, Pazo-Cid R, Arkenau HT, Borg C, Lordick F, Li L, Ding N, Tao A, Shi J, Van Cutsem E. Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study. Journal of clinical oncology, 40:3065-3076, 2022
30. Itami J, Kobayashi K, Mori T, Honma Y, Kubo Y, Murakami N, Omura G, Okuma K, Inaba K, Takahashi K, Kashihara T, Shimizu Y, Takahashi A, Nakayama Y, Matsumoto F, Yoshimoto S, Igaki H. Non-Robustness of Ang’s Risk Classification in Human Papillomavirus-Related Oropharyngeal Squamous Cell Carcinoma in Japanese Patients. Cancers, 14:2442, 2022
31. Kojima T, Hara H, Tsuji A, Yasui H, Muro K, Satoh T, Ogata T, Ishihara R, Goto M, Baba H, Nishina T, Han S, Sakata T, Yatsuzuka N, Doi T, Kato K. First-line pembrolizumab + chemotherapy in Japanese patients with advanced/metastatic esophageal cancer from KEYNOTE-590. Esophagus, 19:683-692, 2022
32. Kurokawa Y, Honma Y, Sawaki A, Naito Y, Iwagami S, Komatsu Y, Takahashi T, Nishida T, Doi T. Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase III trial. Annals of oncology, 33:959-967, 2022
33. Yamaguchi K, Minashi K, Sakai D, Nishina T, Omuro Y, Tsuda M, Iwagami S, Kawakami H, Esaki T, Sugimoto N, Oshima T, Kato K, Amagai K, Hosaka H, Komine K, Yasui H, Negoro Y, Ishido K, Tsushima T, Han S, Shiratori S, Takami T, Shitara K. Phase IIb study of pembrolizumab combined with S-1 + oxaliplatin or S-1 + cisplatin as first-line chemotherapy for gastric cancer. Cancer science, 113:2814-2827, 2022
34. Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, Kawamura O, Kusano M, Kuwano H, Takeuchi H, Toh Y, Doki Y, Naomoto Y, Nemoto K, Booka E, Matsubara H, Miyazaki T, Muto M, Yanagisawa A, Yoshida M. Correction to: Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1 and Part 2. Esophagus, 19:726, 2022
35. Van Cutsem E, Kato K, Ajani J, Shen L, Xia T, Ding N, Zhan L, Barnes G, Kim SB. Tislelizumab versus chemotherapy as second-line treatment of advanced or metastatic esophageal squamous cell carcinoma (RATIONALE 302): impact on health-related quality of life. ESMO open, 7:100517, 2022
36. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Lockhart AC, Arkenau HT, El-Hajbi F, Gupta M, Pfeiffer P, Bhagia P, Cao ZA, Lunceford J, Suryawanshi S, Ayers M, J Marton M, Kato K. T cell-inflamed gene expression profile and PD-L1 expression and pembrolizumab efficacy in advanced esophageal cancer. Future oncology (London, England), 18:2783-2790, 2022
37. Kojima T, Kato K, Hara H, Takahashi S, Muro K, Nishina T, Wakabayashi M, Nomura S, Sato A, Ohtsu A, Doi T. Phase II study of BKM120 in patients with advanced esophageal squamous cell carcinoma (EPOC1303). Esophagus, 19:702-710, 2022
38. Udagawa C, Kuah S, Shimoi T, Kato K, Yoshida T, Nakano MH, Shimo A, Kojima Y, Yoshie R, Tsugawa K, Mushiroda T, Tan EY, Zembutsu H. Replication Study for the Association of Five SNPs Identified by GWAS and Trastuzumab-Induced Cardiotoxicity in Japanese and Singaporean Cohorts. Biological & pharmaceutical bulletin, 45:1198-1202, 2022
39. Hino K, Nishina T, Kajiwara T, Bando H, Nakamura M, Kadowaki S, Minashi K, Yuki S, Ohta T, Hara H, Mizukami T, Moriwaki T, Ohtsubo K, Komoda M, Mitani S, Nagashima F, Kato K, Yamada T, Hasegawa H, Yamazaki K, Yoshino T, Hyodo I. Association of ERBB2 Copy Number and Gene Coalterations With Trastuzumab Efficacy and Resistance in Human Epidermal Growth Factor Receptor 2-Positive Esophagogastric and Gastric Cancer. JCO precision oncology, 6:e2200135, 2022
40. Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA oncology, 8:1447-1455, 2022
41. Shimizu Y, Murakami N, Mori T, Takahashi K, Kubo Y, Yoshimoto S, Honma Y, Nakamura S, Okamoto H, Iijima K, Takahashi A, Kaneda T, Kashihara T, Inaba K, Okuma K, Nakayama Y, Igaki H, Itami J. Clinical impact of p16 positivity in nasopharyngeal carcinoma. Laryngoscope investigative otolaryngology, 7:994-1001, 2022
42. Kawakita D, Nagao T, Takahashi H, Kano S, Honma Y, Hirai H, Saigusa N, Akazawa K, Tani K, Ojiri H, Tsukahara K, Ozawa H, Okami K, Kondo T, Togashi T, Fushimi C, Shimura T, Shimizu A, Okamoto I, Okada T, Imanishi Y, Watanabe Y, Otsuka K, Sakai A, Ebisumoto K, Sato Y, Yamazaki K, Ueki Y, Hanazawa T, Saito Y, Ando M, Matsuki T, Nakaguro M, Sato Y, Urano M, Utsumi Y, Kohsaka S, Saotome T, Tada Y. Survival benefit of HER2-targeted or androgen deprivation therapy in salivary duct carcinoma. Therapeutic advances in medical oncology, 14:17588359221119538, 2022
43. Miyata Y, Murakami N, Honma Y, Mori T, Yoshimoto S, Kashihara T, Takemori M, Nakayama Y, Itami J, Ogo E, Igaki H. Technical report: a high-dose-rate interstitial brachytherapy boost for residual sinonasal undifferentiated carcinoma. Journal of radiation research, 63:879-883, 2022
44. Yamamoto S, Sakakibara N, Hirano H, Morizane C, Honma Y, Hijioka S, Okusaka T, Higashi T, Kawai A. The real-world selection of first-line systemic therapy regimen for metastatic gastroenteropancreatic neuroendocrine neoplasm in Japan. Scientific reports, 12:17601, 2022
45. Kohsaka S, Tada Y, Ando M, Nakaguro M, Shirai Y, Ueno T, Kojima S, Hirai H, Saigusa N, Kano S, Tsukahara K, Togashi T, Ozawa H, Kondo T, Okami K, Takahashi H, Kawakita D, Fushimi C, Suzuki T, Shimizu A, Okamoto I, Okada T, Sato Y, Imanishi Y, Watanabe Y, Sakai A, Ebisumoto K, Sato Y, Urano M, Honma Y, Yamazaki K, Ueki Y, Hanazawa T, Saito Y, Shimura T, Nagao T, Mano H. Identification of novel prognostic and predictive biomarkers in salivary duct carcinoma via comprehensive molecular profiling. NPJ precision oncology, 6:82, 2022
46. Nakagawa K, Sho M, Fujishiro M, Kakushima N, Horimatsu T, Okada KI, Iguchi M, Uraoka T, Kato M, Yamamoto Y, Aoyama T, Akahori T, Eguchi H, Kanaji S, Kanetaka K, Kuroda S, Nagakawa Y, Nunobe S, Higuchi R, Fujii T, Yamashita H, Yamada S, Narita Y, Honma Y, Muro K, Ushiku T, Ejima Y, Yamaue H, Kodera Y. Clinical practice guidelines for duodenal cancer 2021. Journal of gastroenterology, 57:927-941, 2022
47. Miura T, Mitsunaga S, Matsuzaki J, Takizawa S, Kato K, Ochiai A, Ochiya T. Serum microRNAs as new criteria for referral to early palliative care services in treatment-naïve advanced cancer patients. Oncotarget, 13:1341-1349, 2022
48. Saigusa N, Hirai H, Tada Y, Kawakita D, Nakaguro M, Tsukahara K, Kano S, Ozawa H, Kondo T, Okami K, Togashi T, Sato Y, Urano M, Kajiwara M, Shimura T, Fushimi C, Shimizu A, Okamoto I, Okada T, Suzuki T, Imanishi Y, Watanabe Y, Sakai A, Ebisumoto K, Sato Y, Honma Y, Yamazaki K, Ueki Y, Hanazawa T, Saito Y, Takahashi H, Ando M, Kohsaka S, Matsuki T, Nagao T. The Role of the EZH2 and H3K27me3 Expression as a Predictor of Clinical Outcomes in Salivary Duct Carcinoma Patients: A Large-Series Study With Emphasis on the Relevance to the Combined Androgen Blockade and HER2-Targeted Therapy. Frontiers in oncology, 11:779882, 2021
