Annual Report 2022
Department of Gastric Surgery
Takaki Yoshikawa, Yukinori Yamagata, Tsutomu Hayashi, Takeyuki Wada, Kenichi Ishizu, Ryota Sakon
Introduction
Our division provides surgical treatment for patients with gastric cancer, adenocarcinoma of esophago-gastric junction, and gastrointestinal stromal tumors (GIST). The aim of our division is to contribute to human health through treatment and research in this field. Our policy to achieve this goal is to (1) provide high quality treatment for patients, (2) efficiently perform clinical tasks, and (3) contribute to developing treatments through clinical trials and research and provide high quality education.
The Team and What We Do
(1) We conduct daily morning conference to determine the treatment strategy for new patients and those who required any new treatment to provide high quality treatment. All the staff check the endoscopy and CT, and determine the tumor staging based on the uniform criteria, and discuss the treatment strategy for each patient based on the problem when applying the standard treatment and the patient's indication to be a candidate for any clinical trial. Surgical morbidity and hospital stay were dramatically improved after introducing the Enhanced Recovery After Surgery program (ERAS) for perioperative care.
(2) We prepared the medical documents for the patients for the uniform explanation and the printed protocol at the conference room and the outpatient clinic to check the applicability to the clinical trial for an efficient clinical task. We also relieve the physicians and residents from the task of preparing the database.
(3) Our division operated a total 266 cases of gastric cancer, GIST, and others this year (Table 1). Table 2 shows the detailed surgical procedure. The proportion of laparoscopic distal gastrectomy/pylorus-preserving gastrectomy increased annually; from 49% (2017) to 65% (2018), 62% (2019), 62% (2020), 74% (2021), and 87.7% (2022) and that of total/proximal gastrectomy from 12% (2017) to 21% (2018), 44% (2019), 37% (2020), 46% (2021), and 67.8% (2022). Cases of robot assisted laparoscopic gastrectomy, initiated in 2019, accounted for 19 (2020), 36 (2021), and 54 (2022). Conversely, an extended surgery is not rare. We gained experience of numerous cases of D2 plus para-aortic nodal dissection after neoadjuvant chemotherapy for bulky nodal disease or para-aortic nodal metastasis and several left upper abdominal exenteration cases for bulky GIST invading adjacent organs and lower mediastinal dissection through trans-hiatal approach and by right video-assisted trans-thoracic approach for gastric cancer invading the lower esophagus. We can provide any type of surgery for the local control as applicable. Finally, we presented the overall survival curves of 6556 patients who received surgery from 2000 to 2017 (Figure 1, n=6757). The overall 5-year survival rate was 95.6% in stage IA, 93.1% in stage IB, 88.5% in stage IIA, 81.7% in stage IIB, 68.5% in stage IIIA, 56.9% in stage IIIB, 33.2% in stage IIIC, and 17.5% in stage IV following the 15th edition of the Japanese Classification of Gastric Cancer.
Figure 1. Overall survival curves of patients who have undergone resection (2000-2017, n=6757)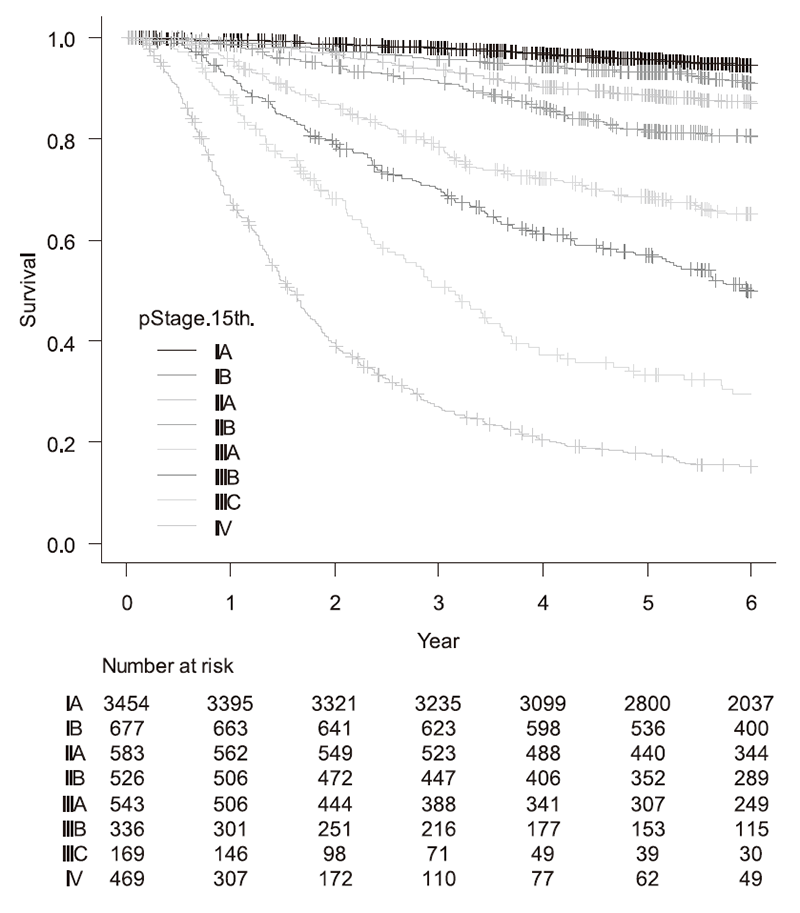

Research Activities
During daily clinical practice, we notice a lot of clinical questions which have not been clarified. To answer many clinical questions, we have been performing many retrospective studies using our database and clinical materials, submitting manuscripts, and reporting in English journals.
Clinical Trials
Our major task is treatment development through clinical trials. We lead many JCOG trials.
JCOG1104 (Primary investigator: Yoshikawa) is a phase III trial to confirm the non-inferiority of 4 courses of S-1 adjuvant chemotherapy to 8 courses S-1 for pathological stage II. A 5-year follow-up was terminated in Mar 2022. We presented the final follow-up results of JCOG1104 at ASCO-GI in January 2023, and submitted the manuscript.
JCOG1711 (Representative investigator: Yoshikawa) is a phase III trial to confirm the non-inferiority of omental preservation to omentectomy for SS/SE gastric cancer. Accrual has been ongoing. Laparoscopic surgery was permitted as a protocol treatment because laparoscopic gastrectomy was established as one of the standard surgeries based on the Japanese phase III trial.
JCOG2301 (Representative investigator: Yoshikawa) is a phase III trial to confirm the superiority of conversion surgery to chemotherapy for stage IV gastric cancer in which a distant metastatic site disappeared by chemotherapy. The trial concept was approved, and the protocol has been prepared.
JCOG1302A (Primary investigator: Hayashi, Representative investigator: Yoshikawa) is an exploratory analysis to examine the prognosis of patients who were enrolled in JCOG1302 to evaluate the accuracy of clinical diagnosis in locally resectable advanced gastric cancer. The result was presented at ASCO-GI in January 2022, and the manuscript has been prepared.
JCOG2212A (Primary investigator: Wada, Representative investigator: Yoshikawa) is a combined analysis of JCOG0001, 0405, and 1002 to evaluate the safety and efficacy of neoadjuvant chemotherapy for bulky or para-aortic nodal disease. This combined analysis aims to clarify the therapeutic value of para-aortic nodal dissection. The trial has been ongoing to collect prognostic data.
Many JCOG trials are conducted in addition to the above-mentioned trials. We continue the accrual of new patients to JCOG1507 phase III (surgery alone vs S-1 for elderly patients with pathological stage II/III gastric cancer), JCOG1509 phase III (surgery followed by adjuvant chemotherapy vs neoadjuvant chemotherapy of S-1/oxaliplatin followed by surgery and adjuvant chemotherapy for patients with SS/SE gastric cancer invading lymph nodes), JCOG1809 phase II (laparoscopic splenic hilar nodal dissection for upper gastric cancer invading the greater curvature), JCOG1902 single-arm phase III (ESD for submucosal gastric cancer in the elderly patients), and JCOG1907 phase III (laparoscopic vs robotic gastrectomy for clinical stage I/IIA gastric cancer). We follow the patients who had enrolled in the clinical trial of JCOG1104 phase III (8 courses S-1 vs 4 courses S-1 for stage II), JCOG1301C randomized phase II (neoadjuvant chemotherapy of S-1/CDDP with or without trastuzumab for HER2 positive gastric cancer invading lymph nodes), and JCOG1401 single arm phase III (laparoscopic total/proximal gastrectomy for stage I).
On the collaborative research with the UK, the Netherlands, Singapore, and Germany, we published several papers in Gastric Cancer, Br J Surg, Sci Rep, and Gut.
This year, we started a global phase III study to confirm the efficacy of durvalumab, a new PDL-1 antibody, in addition to FLOT, a western standard perioperative chemotherapy for stage II/III gastric cancer.
Education
Through open conferences to determine tumor staging and treatment strategy, the trainees could study the standard treatment, the problem for applying the standard therapy, and the investigational treatment. They also could experience the first/second assistance of surgery performed by experienced staff, the scopist of laparoscopic surgery, and even the operator under the instruction of experienced staff. To obtain the certification of the endoscopic surgeon, the trainees could experience the animal training and receive video-review by experienced staff. Following the success of past residents, the chief resident of this year passed the certification of the endoscopic surgeon although the passing rate of the exam was approximately 20%. The passing rate in our department has been over 80% since 2018.
Young surgeons also continued clinical research and published many English papers.
Future Prospects
We will continue the high quality treatment, efficient clinical tasks, clinical trials, clinical research, and education in the field of gastric surgery and contribute to the human being.
List of papers published in 2022
Journal
1. Toriumi T, Terashima M, Mizusawa J, Sato Y, Kurokawa Y, Takiguchi S, Doki Y, Shinohara H, Teshima S, Yasuda T, Ito S, Yoshikawa T, Sano T, Sasako M. Recurrence patterns after curative gastrectomy for pStage II/III gastric cancer: Exploratory analysis of the randomized controlled JCOG1001 trial. European journal of surgical oncology, 49:838-844, 2023
2. Hikage M, Hato S, Uemura K, Yura M, Sato Y, Matsushita H, Cho H, Hiki N, Kunisaki C, Inoue K, Choda Y, Boku N, Yoshikawa T, Katai H, Terashima M. Late complication after gastrectomy for clinical stage I cancer: supplementary analysis of JCOG0912. Surgical endoscopy, 37:2958-2968, 2023
3. Akiyama Y, Katai H, Kitabayashi R, Nunobe S, Koeda K, Yura M, Sato Y, Yoshikawa T, Terashima M. Frequency of lymph node metastasis according to tumor location in clinical T1 early gastric cancer: supplementary analysis of the Japan Clinical Oncology Group study (JCOG0912). Journal of gastroenterology, 58:519-526, 2023
4. Matsuzaki J, Kato K, Oono K, Tsuchiya N, Sudo K, Shimomura A, Tamura K, Shiino S, Kinoshita T, Daiko H, Wada T, Katai H, Ochiai H, Kanemitsu Y, Takamaru H, Abe S, Saito Y, Boku N, Kondo S, Ueno H, Okusaka T, Shimada K, Ohe Y, Asakura K, Yoshida Y, Watanabe SI, Asano N, Kawai A, Ohno M, Narita Y, Ishikawa M, Kato T, Fujimoto H, Niida S, Sakamoto H, Takizawa S, Akiba T, Okanohara D, Shiraishi K, Kohno T, Takeshita F, Nakagama H, Ota N, Ochiya T. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI cancer spectrum, 7:pkac080, 2023
5. Kumazu Y, Hasegawa S, Hayashi T, Yamada T, Watanabe H, Hara K, Shimoda Y, Nakazono M, Nagasawa S, Rino Y, Masuda M, Ogata T, Oshima T, Yoshikawa T. Should the splenic hilar lymph node be dissected for the management of adenocarcinoma of the esophagogastric junction? European journal of surgical oncology, 49:76-82, 2023
6. Naka T, Hashimoto T, Cho H, Tanabe N, Yoshida T, Yatabe Y, Yoshikawa T, Abe S, Sekine S. Sporadic and Familial Adenomatous Polyposis-associated Foveolar-type Adenoma of the Stomach. The American journal of surgical pathology, 47:91-101, 2023
7. Satake H, Lee KW, Chung HC, Lee J, Yamaguchi K, Chen JS, Yoshikawa T, Amagai K, Yeh KH, Goto M, Chao Y, Lam KO, Han SR, Shiratori S, Shah S, Shitara K. Pembrolizumab or pembrolizumab plus chemotherapy versus standard of care chemotherapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma: Asian subgroup analysis of KEYNOTE-062. Japanese journal of clinical oncology, 53:221-229, 2023
8. Yamagata Y, Yoshikawa T, Ishizu K, Tsutsui M, Wada T, Hayashi T. Impact of D2 Gastrectomy for Locally Advanced Gastric Cancer in the Era of Effective Adjuvant Chemotherapy. World journal of surgery, 47:1512-1518, 2023
9. Nishino M, Yoshikawa T, Yura M, Sakon R, Ishizu K, Wada T, Hayashi T, Yamagata Y. Possible candidates for splenic hilar nodal dissection among patients with upper advanced gastric cancer without invasion of the greater curvature. Gastric cancer, 26:460-466, 2023
10. Natsume, Hiroko// Szczepaniak, Kinga// Yamada, Hidetaka// Iwashita, Yuji// Gędek, Marta// Šuto, Jelena// Ishino, Keiko// Kasajima, Rika// Matsuda, Tomonari// Manirakiza, Felix// Nzitakera, Augustin// Wu, Yijia// Xiao, Nong// He, Qiong// Guo, Wenwen// Cai, Zhenming// Ohta, Tsutomu// Szekely, Tıberiu// Kadar, Zoltan// Sekiyama, Akiko// Oshima, Takashi// Yoshikawa, Takaki// Tsuburaya, Akira// Kurono, Nobuhito// Wang, Yaping// Miyagi, Yohei// Gurzu, Simona// Sugimura, Haruhiko . Non-CpG sites preference in G:C > A:T transition of TP53 in gastric cancer of Eastern Europe (Poland, Romania and Hungary) compared to East Asian countries (China and Japan) . Genes and environment : the official journal of the Japanese Environmental Mutagen Society, 45:1, 2023
11. Hagi T, Kurokawa Y, Mizusawa J, Fukagawa T, Katai H, Sano T, Misawa K, Fukushima N, Kawachi Y, Sasako M, Yoshikawa T, Terashima M. Impact of tumor-related factors and inter-institutional heterogeneity on preoperative T staging for gastric cancer. Future oncology (London, England), 18:2511-2519, 2022
12. Kawakami T, Mizusawa J, Hasegawa H, Imazeki H, Kano K, Sato Y, Iwasa S, Takiguchi S, Kurokawa Y, Doki Y, Boku N, Yoshikawa T, Terashima M. Usefulness of an S-1 dosage formula: an exploratory analysis of randomized clinical trial (JCOG1001). Gastric cancer, 25:1073-1081, 2022
13. Kurokawa Y, Doki Y, Mizusawa J, Yoshikawa T, Yamada T, Kimura Y, Takiguchi S, Nishida Y, Fukushima N, Cho H, Kaji M, Hirao M, Sasako M, Terashima M. Five-year follow-up of a randomized clinical trial comparing bursectomy and omentectomy alone for resectable gastric cancer (JCOG1001). The British journal of surgery, 110:50-56, 2022
14. Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, Kochi M, Horinouchi H, Kinoshita T, Yoshikawa T, Muro K, Nishikawa H, Suna H, Kodera Y. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric cancer, 25:619-628, 2022
15. Suzuki K, Yamaguchi T, Kohda M, Tanaka M, Takemura H, Wakita M, Tabe Y, Kato S, Nasu M, Hashimoto T, Mine S, Serizawa N, Tomishima K, Nagahara A, Matsuda T, Yamaji T, Tsugane S, Saito Y, Daiko H, Yoshikawa T, Kato K, Okusaka T, Ochiya T, Yamamoto Y, Yotsui S, Yamamoto T, Yamasaki T, Miyata H, Yasui M, Omori T, Ohkawa K, Ikezawa K, Nakabori T, Sugimoto N, Kudo T, Yoshida K, Ohue M, Nishizawa T. Establishment of preanalytical conditions for microRNA profile analysis of clinical plasma samples. PloS one, 17:e0278927, 2022
16. Sundar R, Barr Kumarakulasinghe N, Huak Chan Y, Yoshida K, Yoshikawa T, Miyagi Y, Rino Y, Masuda M, Guan J, Sakamoto J, Tanaka S, Tan AL, Hoppe MM, Jeyasekharan AD, Ng CCY, De Simone M, Grabsch HI, Lee J, Oshima T, Tsuburaya A, Tan P. Machine-learning model derived gene signature predictive of paclitaxel survival benefit in gastric cancer: results from the randomised phase III SAMIT trial. Gut, 71:676-685, 2022
17. Hayashi T, Yoshikawa T. Optimal surgery for esophagogastric junctional cancer. Langenbeck’s archives of surgery, 407:1399-1407, 2022
18. Kamiya A, Yoshikawa T, Sakon R, Ishizu K, Wada T, Hayashi T, Otsuki S, Yamagata Y, Katai H. Optimal surgery and lymph node metastasis of duodenal bulbar neuroendocrine neoplasms. European journal of surgical oncology, 48:597-603, 2022
19. Terashima M, Sano T, Mizusawa J, Umemura K, Tokunaga M, Omori T, Cho H, Hasegawa Y, Akiyama Y, Tsujimoto H, Kawashima Y, Kawachi Y, Lee SW, Kano K, Hasegawa H, Boku N, Yoshikawa T, Sasako M. Prediction of the peritoneal recurrence via the macroscopic diagnosis of the serosal invasion in patients with gastric cancer: Supplementary analysis of JCOG0110. European journal of surgical oncology, 48:1753-1759, 2022
20. Yamada H, Takeshima H, Fujiki R, Yamashita S, Sekine S, Ando T, Hattori N, Okabe A, Yoshikawa T, Obama K, Katai H, Kaneda A, Ushijima T. ARID1A loss-of-function induces CpG island methylator phenotype. Cancer letters, 532:215587, 2022
21. Cho H, Hashimoto T, Naka T, Yatabe Y, Oda I, Saito Y, Yoshikawa T, Sekine S. Activating KRAS and GNAS mutations in heterotopic submucosal glands of the stomach. Journal of gastroenterology, 57:333-343, 2022
22. Yanagimoto Y, Kurokawa Y, Doki Y, Yoshikawa T, Boku N, Terashima M. Surgical and perioperative treatment strategy for resectable esophagogastric junction cancer. Japanese journal of clinical oncology, 52:417-424, 2022
23. Hayashi T, Yoshikawa T. Reply to: Letter to the Editor "Suprapancreatic nodal dissection should not be uniformly selected in additional gastrectomy for the patients diagnosed as pT1b gastric cancer by endoscopic resection". European journal of surgical oncology, 48:1867-1868, 2022
24. Hayashi T, Yoshikawa T, Ishizu K, Tsutsui M, Wada T, Yamagata Y, Katai H. Suprapancreatic nodal dissection should not be uniformly selected in additional gastrectomy for the patients who diagnosed as pT1b gastric cancer by endoscopic resection. European journal of surgical oncology, 48:1785-1789, 2022
25. Silva ANS, Saito Y, Yoshikawa T, Oshima T, Hayden JD, Oosting J, Earle S, Hewitt LC, Slaney HL, Wright A, Inam I, Langley RE, Allum WH, Nankivell MG, Hutchins G, Cunningham D, Grabsch HI. Author response to: Increasing frequency of gene copy number aberrations is associated with immunosuppression and predicts poor prognosis in gastric adenocarcinoma. The British journal of surgery, 109:e106, 2022
26. Oshima T, Tsuburaya A, Yoshida K, Yoshikawa T, Miyagi Y, Rino Y, Masuda M, Guan J, Tan P, Grabsch HI, Sakamoto J, Tanaka S. Gastric cancer biomarker analysis in patients treated with different adjuvant chemotherapy regimens within SAMIT, a phase III randomized controlled trial. Scientific reports, 12:8509, 2022
27. Kamiya A, Hayashi T, Sakon R, Ishizu K, Wada T, Otsuki S, Yamagata Y, Katai H, Yoshikawa T. Long-term postoperative pneumonia in elderly patients with early gastric cancer. BMC surgery, 22:220, 2022
28. Kakeji Y, Ishikawa T, Suzuki S, Akazawa K, Irino T, Miyashiro I, Ono H, Suzuki H, Tanabe S, Kadowaki S, Muro K, Fukagawa T, Nunobe S, Wada T, Katai H, Kodera Y. A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001-2013). Gastric cancer, 25:1082-1093, 2022
29. Aoyama T, Yoshikawa T, Ida S, Cho H, Sakamaki K, Ito Y, Fujitani K, Takiguchi N, Kawashima Y, Nishikawa K, Nunobe S, Hiki N. Effects of perioperative eicosapentaenoic acid-enriched oral nutritional supplement on the long-term oncological outcomes after total gastrectomy for gastric cancer. Oncology letters, 23:151, 2022
30. Yamada T, Hayashi T, Fujikawa H, Kumazu Y, Nagasawa S, Nakazono M, Kano K, Hara K, Watanabe H, Komori K, Shimoda Y, Takahashi K, Ogata T, Oshima T, Yoshikawa T. Feasibility and Safety of Oral Nutritional Supplementation with High-Density Liquid Diet After Total Gastrectomy for Gastric Cancer. World journal of surgery, 46:2433-2439, 2022
31. Kamiya A, Hayashi T, Sakon R, Ishizu K, Wada T, Otsuki S, Yamagata Y, Katai H, Yoshikawa T. Prognostic Impact of Long-term Postoperative Pneumonia in Elderly Patients with Early Gastric Cancer. Journal of Cancer, 13:2905-2911, 2022
32. Komori K, Kano K, Aoyama T, Hara K, Nagasawa S, Nakazono M, Shimoda Y, Maezawa Y, Kumazu Y, Kawabe T, Numata M, Hayashi T, Yamada T, Tamagawa H, Sato T, Cho H, Yukawa N, Rino Y, Yoshikawa T, Ogata T, Oshima T. Clinical Impact of Surgical Sarcopenia on Long-term Survival. Anticancer research, 42:4545-4552, 2022
33. Kawakami H, Nishikawa K, Shimokawa T, Fujitani K, Tamura S, Endo S, Kobayashi M, Kawada J, Kurokawa Y, Tsuburaya A, Yoshikawa T, Sakamoto J, Satoh T, On Behalf Of The Herbis-Herbis-A And XParTS Ii Study Investigators. Histology Classification Highlights Differences in Efficacy of S-1 versus Capecitabine, in Combination with Cisplatin, for HER2-Negative Unresectable Advanced or Recurrent Gastric Cancer with Measurable Disease. Cancers, 14:5673, 2022
