Annual Report 2022
Department of Hematology
Koji Izutsu, Tatsuya Suzuki, Wataru Munakata, Suguru Fukuhara, Noriko Iwaki, Shinichi Makita, Hirokazu Sasaki, Daiki Hattori, Haruhi Makino
Introduction
The Department of Hematology of the National Cancer Center Hospital is dedicated to supporting patients with hematologic malignancies including leukemia, lymphoma, and myeloma by offering state-of-the-art diagnosis and treatment options and developing novel treatment and diagnostic methods through clinical trials and translational research. Our Lymphoma Program has a history of pioneering clinical research on adult T-cell leukemia/lymphoma and other lymphoid malignancies, and has been leading the development of novel agents and cellular therapies for hematologic malignancies in Japan.
The Team and What We Do
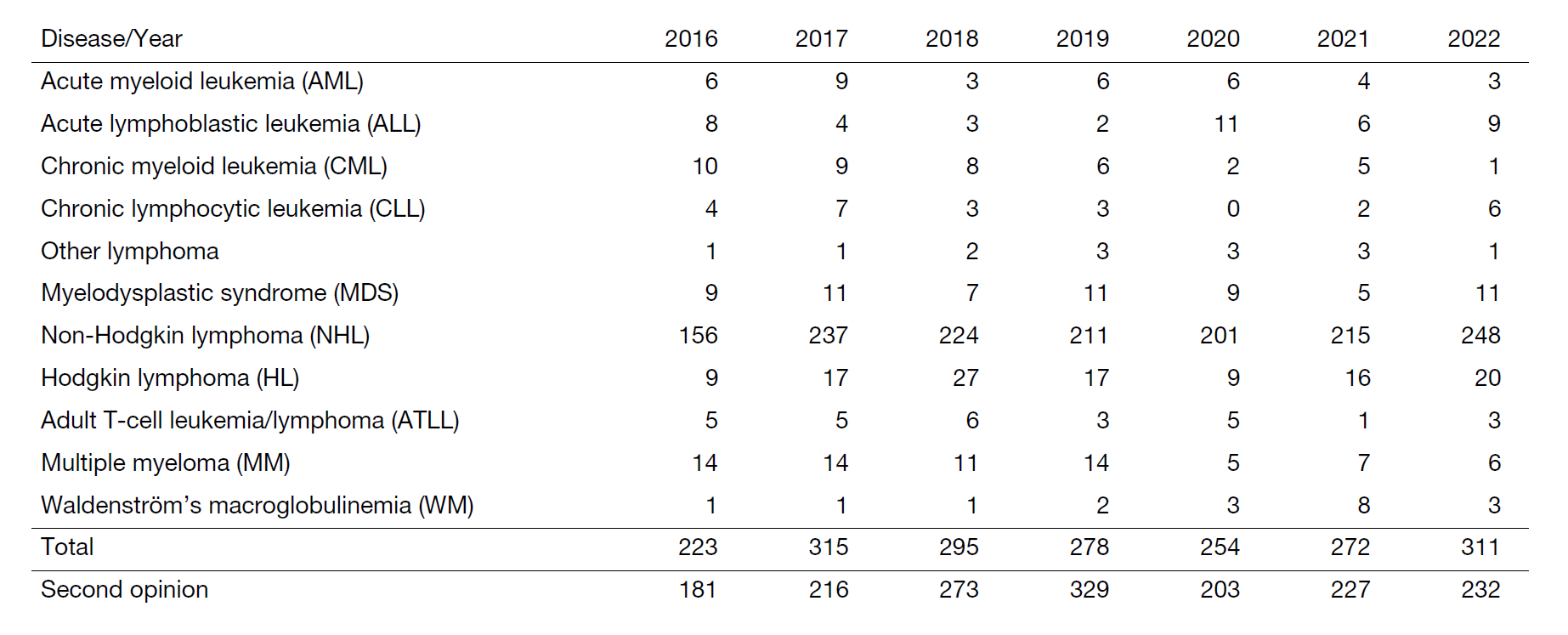
We offer the most advanced diagnostic procedures and care for patients with hematologic malignancies (e.g., leukemia, lymphoma, myelodysplastic syndrome, and multiple myeloma), both on an outpatient and inpatient basis (Table 1). The medical treatments we offer include chemotherapy, immunotherapy, and chimeric antigen receptor (CAR) T-cell therapy. We treat patients in collaboration with experts from our center's Department of Hematopoietic Stem Cell Transplantation, Department of Radiation Oncology, and Department of Experimental Therapeutics. Moreover, experts at our center's Department of Pathology and Department of Clinical Laboratories offer state-of-the-art diagnosis to guide treatment.
Research Activities
We have launched a tumor sample banking system for hematological malignancies for future research use. These samples include tumor DNA derived from bone marrow, peripheral blood, and other tumor tissue obtained through core needle biopsy. We are conducting clinical and clinical-pathology research for hematological malignancies, especially for malignant lymphoma. In 2018, in collaboration with other departments, we launched the MASTER KEY HEM registration study for rare hematologic malignancies incorporating biomarkers. In 2022, we launched an investigator-initiated clinical trial of a BTK inhibitor for secondary central nervous system lymphoma. Our department is devoted to activities of the leading clinical study groups on hematological malignancies in Japan, namely, the Japan Clinical Oncology Group - Lymphoma Study Group (JCOG LSG) and the Japan Adult Leukemia Study Group (JALSG). This year, we authored or coauthored 27 articles related to hematological malignancies.
Clinical Trials
In 2022, we conducted 59 corporate-sponsored clinical trials, including many global studies, one first-in-human study, and 6 new studies that started from April 2022 to March 2023. We also conducted several investigator-initiated clinical trials of JCOG and JALSG.
Education
We have been offering a training program for young hematologists and/or oncologists. Many of our graduates are actively engaged in hematology and oncology societies.
Future Prospects
The Department of Hematology will continue to address unmet needs in the treatment of hematological malignancies. We are focusing on developing treatments for rare hematologic malignancies including rare subtypes of lymphoma. Our future plans include genome-based medicine. Information obtained from genomic tests has become imperative in the diagnosis according to the current WHO classification of hematologic malignancies, prognosis prediction, and for guiding selection of molecular-targeted agents.
List of papers published in 2022
Journal
1. Casulo C, Santoro A, Cartron G, Ando K, Munoz J, Le Gouill S, Izutsu K, Rule S, Lugtenburg P, Ruan J, Arcaini L, Casadebaig ML, Fox B, Kilavuz N, Rettby N, Dell’Aringa J, Taningco L, Delarue R, Czuczman M, Witzig T. Durvalumab as monotherapy and in combination therapy in patients with lymphoma or chronic lymphocytic leukemia: The FUSION NHL 001 trial. Cancer reports (Hoboken, N.J.), 6:e1662, 2023
2. Izutsu K, Makita S, Nosaka K, Yoshimitsu M, Utsunomiya A, Kusumoto S, Morishima S, Tsukasaki K, Kawamata T, Ono T, Rai S, Katsuya H, Ishikawa J, Yamada H, Kato K, Tachibana M, Kakurai Y, Adachi N, Tobinai K, Yonekura K, Ishitsuka K. An open-label, single-arm phase 2 trial of valemetostat for relapsed or refractory adult T-cell leukemia/lymphoma. Blood, 141:1159-1168, 2023
3. Fukuhara N, Maruyama D, Hatake K, Nagai H, Makita S, Kamezaki K, Uchida T, Kusumoto S, Kuroda J, Iriyama C, Yanada M, Tsukamoto N, Suehiro Y, Minami H, Garcia-Vargas J, Childs BH, Yasuda M, Masuda S, Tsujino T, Terao Y, Tobinai K. Safety and antitumor activity of copanlisib in Japanese patients with relapsed/refractory indolent non-Hodgkin lymphoma: a phase Ib/II study. International journal of hematology, 117:100-109, 2023
4. Rai S, Kim WS, Ando K, Choi I, Izutsu K, Tsukamoto N, Yokoyama M, Tsukasaki K, Kuroda J, Ando J, Hidaka M, Koh Y, Shibayama H, Uchida T, Yang DH, Ishitsuka K, Ishizawa K, Kim JS, Lee HG, Minami H, Eom HS, Kurosawa M, Lee JH, Lee JS, Lee WS, Nagai H, Shindo T, Yoon DH, Yoshida S, Gillings M, Onogi H, Tobinai K. Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: phase IIb results. Haematologica, 108:811-821, 2023
5. Imaizumi Y, Iwanaga M, Nosaka K, Ishitsuka K, Ishizawa K, Ito S, Amano M, Ishida T, Uike N, Utsunomiya A, Ohshima K, Tanaka J, Tokura Y, Tobinai K, Watanabe T, Uchimaru K, Tsukasaki K. Validation of the iATL-PI prognostic index in therapeutic decision-making for patients with smoldering and chronic ATL: a multicenter study. International journal of hematology, 117:206-215, 2023
6. Kato K, Fujii N, Makita S, Goto H, Kanda J, Shimada K, Akashi K, Izutsu K, Teshima T, Fukuda N, Sumitani T, Nakamura S, Sumi H, Shimizu S, Kakurai Y, Yoshikawa K, Tobinai K, Usui N, Hatake K. A phase 2 study of axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma in Japan: 1-year follow-up and biomarker analysis. International journal of hematology, 117:409-420, 2023
7. Abramson JS, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, Ibrahimi S, Mielke S, Mutsaers P, Hernandez-Ilizaliturri F, Izutsu K, Morschhauser F, Lunning M, Crotta A, Montheard S, Previtali A, Ogasawara K, Kamdar M. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood, 141:1675-1684, 2023
8. Munakata W, Ando K, Yokoyama M, Fukuhara N, Yamamoto K, Fukuhara S, Ohmachi K, Mishima Y, Ichikawa S, Ogiya D, Aoi A, Hatsumichi M, Tobinai K. Long-term safety profile of tirabrutinib: final results of a Japanese Phase I study in patients with relapsed or refractory B-cell malignancies. International journal of hematology, 117:553-562, 2023
9. Song Y, Tilly H, Rai S, Zhang H, Jin J, Goto H, Terui Y, Shin HJ, Kim WS, Cao J, Feng J, Eom HS, Kim TM, Tsai XC, Gau JP, Koh H, Zhang L, Song Y, Yang Y, Li W, Huang H, Ando K, Sharman JP, Sehn LH, Bu L, Wang X, Jiang Y, Hirata J, Lee C, Zhu J, Izutsu K. Polatuzumab vedotin in previously untreated DLBCL: an Asia subpopulation analysis from the phase 3 POLARIX trial. Blood, 141:1971-1981, 2023
10. Kim WS, Fukuhara N, Yoon DH, Yamamoto K, Uchida T, Negoro E, Izutsu K, Terui Y, Nakajima H, Ando K, Suehiro Y, Kang HJ, Ko PS, Nagahama F, Sonehara Y, Nagai H, Tien HF, Kwong YL, Tobinai K. Darinaparsin in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of an Asian Phase 2 Study. Blood advances, 7:4903-4912,2023
11. Maeshima AM, Taniguchi H, Furukawa H, Hattori D, Sasaki H, Makita S, Iwaki N, Fukuhara S, Munakata W, Izutsu K. Diagnostic clues of BCL2-negative, faint, or controversial follicular lymphomas: a study of 103 cases. Human pathology, 135:84-92, 2023
12. Hosono N, Chi S, Yamauchi T, Fukushima K, Shibayama H, Katagiri S, Gotoh A, Eguchi M, Morishita T, Ogasawara R, Kondo T, Yanada M, Yamamoto K, Kobayashi T, Kuroda J, Usuki K, Utsu Y, Yoshimitsu M, Ishitsuka K, Ono T, Takahashi N, Iyama S, Kojima K, Nakamura Y, Fukuhara S, Izutsu K, Abutani H, Yamauchi N, Yuda J, Minami Y. Clinical utility of genomic profiling of AML using paraffin-embedded bone marrow clots: HM-SCREEN-Japan 01. Cancer science, 114:2098-2108, 2023
13. Tao K, Inamoto Y, Furukawa H, Hosoba R, Takeda W, Maeshima A, Aoki J, Ito A, Tanaka T, Kim SW, Makita S, Fukuhara S, Kogure Y, Kataoka K, Izutsu K, Fukuda T. Romidepsin-induced durable remission for relapsed nodal peripheral T-cell lymphoma with T follicular helper phenotype after allogeneic hematopoietic cell transplantation. International journal of hematology, 118:292-298,2023
14. Yachida T, Matsuda T, Sakamoto T, Nakajima T, Kakugawa Y, Maeshima AM, Taniguchi H, Kushima R, Tobinai K, Kobara H, Masugata H, Masaki T, Saito Y. Endoscopic features of colorectal lymphoma according to histological type. JGH open, 6:257-262, 2022
15. Fukuhara N, Suehiro Y, Kato H, Kusumoto S, Coronado C, Rappold E, Zhao W, Li J, Gilmartin A, Izutsu K. Parsaclisib in Japanese patients with relapsed or refractory B-cell lymphoma (CITADEL-111): A phase Ib study. Cancer science, 113:1702-1711, 2022
16. Sekiguchi N, Rai S, Munakata W, Suzuki K, Handa H, Shibayama H, Endo T, Terui Y, Iwaki N, Fukuhara N, Tatetsu H, Iida S, Ishikawa T, Iguchi D, Izutsu K. Two-year outcomes of tirabrutinib monotherapy in Waldenström’s macroglobulinemia. Cancer science, 113:2085-2096, 2022
17. Savage KJ, Horwitz SM, Advani R, Christensen JH, Domingo-Domenech E, Rossi G, Morschhauser F, Alpdogan O, Suh C, Tobinai K, Shustov A, Trneny M, Yuen S, Zinzani PL, Trümper L, Ilidge T, O’Connor OA, Pro B, Miao H, Bunn V, Fenton K, Fanale M, Puhlmann M, Iyer S. Role of stem cell transplant in CD30+ PTCL following frontline brentuximab vedotin plus CHP or CHOP in ECHELON-2. Blood advances, 6:5550-5555, 2022
18. Handa H, Cheong JW, Onishi Y, Iida H, Kobayashi Y, Kim HJ, Chiou TJ, Izutsu K, Tsukurov O, Zhou X, Faessel H, Yuan Y, Sedarati F, Faller DV, Kimura A, Wu SJ. Pevonedistat in East Asian patients with acute myeloid leukemia or myelodysplastic syndromes: a phase 1/1b study to evaluate safety, pharmacokinetics and activity as a single agent and in combination with azacitidine. Journal of hematology & oncology, 15:56, 2022
19. Fukuhara S, Oshikawa-Kumade Y, Kogure Y, Shingaki S, Kariyazono H, Kikukawa Y, Koya J, Saito Y, Tabata M, Yoshifuji K, Mizuno K, Miyagi-Maeshima A, Matsushita H, Sugiyama M, Ogawa C, Inamoto Y, Fukuda T, Sugano M, Yamauchi N, Minami Y, Hirata M, Yoshida T, Kohno T, Kohsaka S, Mano H, Shiraishi Y, Ogawa S, Izutsu K, Kataoka K. Feasibility and clinical utility of comprehensive genomic profiling of hematological malignancies. Cancer science, 113:2763-2777, 2022
20. Utsunomiya A, Izutsu K, Jo T, Yoshida S, Tsukasaki K, Ando K, Choi I, Imaizumi Y, Kato K, Kurosawa M, Kusumoto S, Miyagi T, Ohtsuka E, Sasaki O, Shibayama H, Shimoda K, Takamatsu Y, Takano K, Yonekura K, Makita S, Taguchi J, Gillings M, Onogi H, Tobinai K. Oral histone deacetylase inhibitor tucidinostat (HBI-8000) in patients with relapsed or refractory adult T-cell leukemia/lymphoma: Phase IIb results. Cancer science, 113:2778-2787, 2022
21. Hatta S, Fukuhara S, Fujino T, Saito Y, Ito Y, Makita S, Munakata W, Suzuki T, Maruyama D, Kusumoto M, Izutsu K. The role of surveillance computed tomography in patients with follicular lymphoma. Therapeutic advances in hematology, 13:1-11, 2022
22. Makita S, Yamamoto G, Maruyama D, Asano-Mori Y, Kaji D, Ananthakrishnan R, Ogasawara K, Stepan L, Schusterbauer C, Rettby N, Hasskarl J, Izutsu K. Phase 2 results of lisocabtagene maraleucel in Japanese patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Cancer medicine, 11:4889-4899, 2022
23. Yoshimitsu M, Ando K, Ishida T, Yoshida S, Choi I, Hidaka M, Takamatsu Y, Gillings M, Lee GT, Onogi H, Tobinai K. Oral histone deacetylase inhibitor HBI-8000 (tucidinostat) in Japanese patients with relapsed or refractory non-Hodgkin’s lymphoma: phase I safety and efficacy. Japanese journal of clinical oncology, 52:1014-1020, 2022
24. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, Ibrahimi S, Mielke S, Mutsaers P, Hernandez-Ilizaliturri F, Izutsu K, Morschhauser F, Lunning M, Maloney DG, Crotta A, Montheard S, Previtali A, Stepan L, Ogasawara K, Mack T, Abramson JS. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet (London, England), 399:2294-2308, 2022
25. Morschhauser F, Nastoupil L, Feugier P, Schiano de Colella JM, Tilly H, Palomba ML, Bachy E, Fruchart C, Libby EN, Casasnovas RO, Flinn IW, Haioun C, Maisonneuve H, Ysebaert L, Bartlett NL, Bouabdallah K, Brice P, Ribrag V, Le Gouill S, Daguindau N, Guidez S, Pica GM, García-Sancho AM, López-Guillermo A, Larouche JF, Ando K, Gomes da Silva M, André M, Kalung W, Sehn LH, Izutsu K, Cartron G, Gkasiamis A, Crowe R, Xerri L, Fowler NH, Salles G. Six-Year Results From RELEVANCE: Lenalidomide Plus Rituximab (R(2)) Versus Rituximab-Chemotherapy Followed by Rituximab Maintenance in Untreated Advanced Follicular Lymphoma. Journal of clinical oncology, 40:3239-3245, 2022
26. Goto H, Izutsu K, Ennishi D, Mishima Y, Makita S, Kato K, Hanaya M, Hirano S, Narushima K, Teshima T, Nagai H, Ishizawa K. Zandelisib (ME-401) in Japanese patients with relapsed or refractory indolent non-Hodgkin’s lymphoma: an open-label, multicenter, dose-escalation phase 1 study. International journal of hematology, 116:911-921, 2022
27. Abramson JS, Johnston PB, Kamdar M, Ibrahimi S, Izutsu K, Arnason J, Glass B, Mutsaers P, Lunning M, Braverman J, Liu FF, Crotta A, Montheard S, Previtali A, Guo S, Shi L, Solomon SR. Health-related quality of life with lisocabtagene maraleucel vs standard of care in relapsed or refractory LBCL. Blood advances, 6:5969-5979, 2022
