Annual Report 2022
Clinical Research Support Office
- Clinical Research Coordinating Division
Hideki Ueno, Chie Miyano, Kikue Hasegawa, Asako Sakamoto, Emi Yasuda, Sho Murata, Mari Takahashi, Hiroko Kawaguchi, Shinobu Araki, Chiharu Nakano, Asako Kamikawa, Setsuko Kamizaki, Yoshimi Yamaguchi, Yumiko Ikuno, Kimiyo Yoshii, Hiroko Takagi, Maki Takayasu, Aya Matsumoto, Yukako Takasaki, Ran Obara, Harue Ui, Tamami Yamano, Kayo Mukai, Nahoko Hiraoka, Tomomi Tsuchiya, Hatsuki Ohno, Noriko Makita, Chikano Yashiro, Eri Yanagisawa, Yukari Nishiyama, Azumi Seko, Ai Sekido, Rie Goto, Tomoko Morita, Marie Komagata, Miki Shiraiwa, Aya Shiroichi, Anna Yashima, Miki Tamaki, Saki Yoshizawa, Satsuki Otani, Yumi Nagashima, Kohe Yamashita, Chiaki Kanemoto, Yumi Tada, Ayaka Tanaka, Ritsuko Yamamoto, Kazumi Higuchi, Ai Kobayakawa, Maya Niigawa, Hiroe Takayama, Mari Kondo, Tomoko Jinbo, Shiho Isobe, Junko Kojima, Yoko Iino, Fuyumi Oki, Nishi Eri, Haruna Koizumi, Chie Motegi, Kimiko Sega, Katsuaki Imaizumi, Nobuko Ushirozawa, Yoshiko Kanazu, Yoko Ebihara, Harumi Maruno, Junko Horie, Eiko Mimata, Kaori Matsushita, Yuriko Shinotsuka, Yuki Sezaki, Satoko Murakami, Chiaki Yamamoto, Ken Kato, Teiko Yamane, Keiko Wakakuwa, Keiko Shimo, Mayumi Ikeda, Satomi Nakamori, Harumi Mochizuki, Riko Tamura, Sayaka Oba, Azusa Komada
- Research Management Division
Kenichi Nakamura, Natsuko Okita, Tomomi Hata, Tetsuya Sasaki, Makiko Watanabe, Takako Hitomi, Mitsumi Terada, Hisahiro Ito, Naoko Matsui, Mamiko Kawasaki, Hitomi Okuma, Satoshi Kawashima, Masahiko Ichimura, Chiharu Mizoguchi, Yayoi Ando, Hitoshi Ozawa, Naoko So, Masako Inaba, Reiko Isomura, Mamiko Nakata, Eri Tsutui, Sawako Tomatsuri, Sachie Kawabata, Eiko Yorikane, Yoshie Shuda, Kenta Anjo, Kaori Izumino, Satoshi Azuma, Kazumi Ono, Hiroshi Katayama, Tomoko Kataoka, Keisuke Kanato, Keita Sasaki, Yuta Sekino, Yusuke Sano, Ryosuke Kita, Hideki Masai, Kaori Umegai, Junki Mizusawa, Gakuto Ogawa, Ryunosuke Machida, Shun Sadachi, Ryo Kitabayashi, Taro Shibata, Aya Kuchiba, Shogo Nomura, Akihiro Hirakawa, Kohei Uemura, Masayuki Yokoyama, Riku Kajikawa, Naomi Konishi, Masashi Mikami, Shintaro Iwamoto, Yuki Konda
- Data Management Division
Haruhiko Fukuda, Harumi Kaba, Hiromi Katsuki, Yayoi Mitsumori, Tomoko Kojima, Mikio Mori, Nobuko Okamura, Takashi Makiuchi, Keiko Ohata, Yukari Hoshina, Masahisa Kamikura, Yukari Nagasaka, Eiko Sayama, Eru Adachi, Kazumi Kurishita, Yuki Kazumi, Ako Toyoda, Kayo Sawaki, Yoko Kai, Tomomi Tsuchiya, Keiko Suto
Introduction
The Clinical Research Support Office supports clinical research conducted under the leadership of investigators in the National Cancer Center Hospital (NCCH). Support activities include protocol writing, central/local data management, statistical design and analysis, in-house/on-site monitoring, audits, patient recruitment, and other coordinating jobs.
The Team and What We Do
- Clinical Research Coordinating Division
The Clinical Research Coordinating Section and the Clinical Trial Administration Section support a lot of industry-sponsored registration-directed trials as well as investigator-initiated registration-directed trials.
The Biobank and Translational Research Support Section has routinely obtained informed consent to participate as an NCC biobank (NCCBB) donor from patients who consult with the NCCH for the first time. CRCs in this section coordinate translational research in several ways, such as assistance for registration for clinical trials, logistics for pathological specimens, data collection for case report forms, and coordination between sections.
- Research Management Division
The Research Management Division is in charge of central research support functions: i) International clinical trial management, ii) Investigator-initiated registration-directed trial (Chiken) management, iii) Monitoring and consultation, iv) Multi-institutional clinical trial support, v) Biostatistics, vi) Medical device and Pharmaceutical affairs consultation. The division has the capability to support various types of clinical trials covering both early phase ones including first-in-human trials and late phase multi-institutional trials. For the early phase trials, the division mainly offers comprehensive study management, site visit monitoring and safety information management. One of the strengths of the division is that it can coordinate not only domestic trials but also international investigator-initiated registration-directed trials. The multi-institutional trial support function works as the Japan Clinical Oncology Group (JCOG) Operations Office which engages in protocol development, manuscript drafting, study coordination, etc., for mainly late phase trials.
- Data Management Division
The Data Management Division is responsible for central data management and in-house study monitoring in investigator-initiated clinical trials for cancer therapeutic development. The Data Management Section supports early phase cancer trials mainly for drug development including registration trials which are led by physicians in the NCCH. The Multi-institutional Data Management Section supports mostly late development multi-modality multi-institutional phase II or phase III trials for adult solid cancer conducted by the Japan Clinical Oncology Group (JCOG).
Table 1. Supported Trials in the Clinical Research Coordinating Division in FY 2022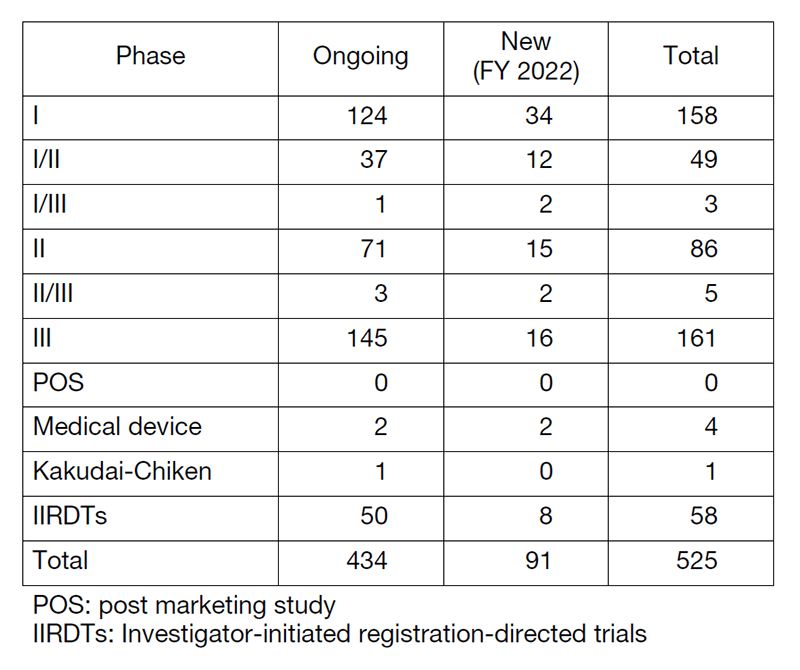

Clinical Trials
- Clinical Research Coordinating Division
The number of registration-directed clinical trials is increasing year by year, and the division supported 525 registration-directed trials including 58 investigator-initiated registration-directed trials in FY 2022 (Table 1).
- Research Management Division
The priority area of the division is genomic medicine, rare cancer and international trials. As of the end of the fiscal year 2021, the Research Management Division supported 21 investigator-initiated registration-directed trials, 14 clinical trials using the Advanced Medical Care system and three trials under patient-proposed health services. This division has been in charge of the coordinating office of rare cancer registry study with basket sub-studies (MASTER KEY Project). This division also supports an international investigator-initiated registration-directed trial (PATHWAY trial), and 3 international investigator-initiated research.
The Data Management Section supports 14 IND trials (2 open, 4 in preparation, 8 on follow-up) and 10 non-IND studies (8 open, 1 in preparation, 1 on follow-up). The Multi-institutional Data Management Section supports 104 non-IND trials (48 open, 17 in preparation, 39 on follow-up) as the JCOG Data Center.
Education
- Clinical Research Coordinating Division
The staff members received not only day-to-day on-the-job training but also in-house educational seminars in order to learn how to support clinical trials including investigator-initiated registration-directed trials.
- Research Management Division
The staff members received not only day-to-day on-the-job training but also various seminars related to clinical trials held within the National Cancer Center or scientific meetings in order to learn how to support clinical trials including investigator-initiated registration trials.
- Data Management Division
Data managers in the division are encouraged to attend the various seminars related to research ethics and clinical trials held within the National Cancer Center, in-house lectures organized by the Clinical Research Support Office, scientific meetings and seminars organized by the Japan Society of Clinical Trials and Research or the Ministry of Health, Labour and Welfare and so on. The division is also continuously promoting on-the-job training of data managers to teach them clinical trial methodology, data management and research ethics.
Future Prospects
- Clinical Research Coordinating Division
The number of supported clinical trials is increasing as previously described, and the supported area covered by CRCs will be expanded to include not only registration trials but also other investigator-initiated clinical trials. Therefore, expansion of CRC staff members is highly anticipated. In view of the plan for the NCCH, all members of this office will work together to contribute to reinforcing clinical research capabilities of the NCCH and to making this office a valuable unit for all members of our hospital.
The members of the Biobank and Translational Research Support Section received seminars which are related to clinical research ethics, etc. The section informs and educates investigators of the NCC about the NCC biobank and translational research periodically through the NCC University. As a future direction, the section will improve the quality of the NCC biobank’s informatics and storage of serum. The section also aims to establish a support system for higher quality and quantity.
- Research Management Division
Since the number of investigator-initiated registration-directed trials has increased, reinforcement of staff resources is urgently needed. In response to this increase, this division will reinforce the support function for various clinical trials including international clinical trials, investigator-initiated registration-directed trials, Advanced Medical Care system trials, patient-proposed health services trials, and a new type of clinical trial (platform trials). Since 2023, two investigator-initiated registration-directed trials utilizing decentralized clinical trial elements have been under preparation. This division will also establish optimal ways to address the Clinical Research Act and revised Ethical Guidelines.
- Data Management Division
The Data Management Division has introduced a web-based electronic data capturing (EDC) system and is promoting standardization of all aspects of data management, such as data formats, case report forms and monitoring reports for increased data integrity, and cost effectiveness of day-to-day work.
List of papers published in 2022
Journal
1. Mizusawa J, Ohba A, Ozaka M, Katayama H, Okusaka T, Kobayashi S, Ikeda M, Terashima T, Sasahira N, Okano N, Miki I, Kaneko T, Mizuno N, Todaka A, Furukawa M, Kajiura S, Kataoka T, Fukuda H, Furuse J, Ueno M, Hepatobiliary and Pancreatic Oncology Group of Japan Clinical Oncology Group. Protocol of a randomized phase II/III study of gemcitabine plus nab-paclitaxel combination therapy versus modified FOLFIRINOX versus S-IROX for metastatic or recurrent pancreatic cancer: JCOG1611 (GENERATE). Japanese Journal of Clinical Oncology, 53:80-84, 2023
2. Nihei K, Minashi K, Yano T, Shimoda T, Fukuda H, Muto M. Final Analysis of Diagnostic Endoscopic Resection Followed by Selective Chemoradiotherapy for Stage I Esophageal Cancer: JCOG0508. Gastroenterology, 164:296-299.e2, 2023
3. Kadota T, Hasuike N, Ono H, Boku N, Mizusawa J, Oda I, Oyama T, Horiuchi Y, Hirasawa K, Yoshio T, Minashi K, Takizawa K, Nakamura K, Muto M. Clinical factors associated with noncurative endoscopic submucosal dissection for the expanded indication of intestinal-type early gastric cancer: Post hoc analysis of a multi-institutional, single-arm, confirmatory trial (JCOG0607). Digestive endoscopy, 35:494-502, 2023
4. Mishima K, Nishikawa R, Narita Y, Mizusawa J, Sumi M, Koga T, Sasaki N, Kinoshita M, Nagane M, Arakawa Y, Yoshimoto K, Shibahara I, Shinojima N, Asano K, Tsurubuchi T, Sasaki H, Asai A, Sasayama T, Momii Y, Sasaki A, Nakamura S, Kojima M, Tamaru JI, Tsuchiya K, Gomyo M, Abe K, Natsumeda M, Yamasaki F, Katayama H, Fukuda H. Randomized phase III study of high-dose methotrexate and whole-brain radiotherapy with/without temozolomide for newly diagnosed primary CNS lymphoma: JCOG1114C. Neuro-oncology, 25:687-698, 2023
5. Toriumi T, Terashima M, Mizusawa J, Sato Y, Kurokawa Y, Takiguchi S, Doki Y, Shinohara H, Teshima S, Yasuda T, Ito S, Yoshikawa T, Sano T, Sasako M. Recurrence patterns after curative gastrectomy for pStage II/III gastric cancer: Exploratory analysis of the randomized controlled JCOG1001 trial. European journal of surgical oncology, 49:838-844, 2023
6. Ura T, Hironaka S, Tsubosa Y, Mizusawa J, Kato K, Tsushima T, Fushiki K, Chin K, Tomori A, Okuno T, Matsushita H, Kojima T, Doki Y, Kusaba H, Fujitani K, Seki S, Kitagawa Y. Early tumor shrinkage and depth of response in patients with metastatic esophageal cancer treated with 2-weekly docetaxel combined with cisplatin plus fluorouracil: an exploratory analysis of the JCOG0807. Esophagus, 20:272-280, 2023
7. Hikage M, Hato S, Uemura K, Yura M, Sato Y, Matsushita H, Cho H, Hiki N, Kunisaki C, Inoue K, Choda Y, Boku N, Yoshikawa T, Katai H, Terashima M. Late complication after gastrectomy for clinical stage I cancer: supplementary analysis of JCOG0912. Surgical endoscopy, 37:2958-2968, 2023
8. Ozaka M, Nakachi K, Kobayashi S, Ohba A, Imaoka H, Terashima T, Ishii H, Mizusawa J, Katayama H, Kataoka T, Okusaka T, Ikeda M, Sasahira N, Miwa H, Mizukoshi E, Okano N, Mizuno N, Yamamoto T, Komatsu Y, Todaka A, Kamata K, Furukawa M, Fujimori N, Katanuma A, Takayama Y, Tsumura H, Fukuda H, Ueno M, Furuse J. A randomised phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel for locally advanced pancreatic cancer (JCOG1407). European journal of cancer (Oxford, England : 1990), 181:135-144, 2023
9. Ishiki H, Kikawa Y, Terada M, Mizusawa J, Honda M, Iwatani T, Mizutani T, Mori K, Nakamura N, Miyaji T, Yamaguchi T, Ando M, Nakamura K, Fukuda H, Kiyota N. Patient-reported outcome and quality of life research policy: Japan Clinical Oncology Group (JCOG) policy. Japanese journal of clinical oncology, 53:195-202, 2023
10. Akiyama Y, Katai H, Kitabayashi R, Nunobe S, Koeda K, Yura M, Sato Y, Yoshikawa T, Terashima M. Frequency of lymph node metastasis according to tumor location in clinical T1 early gastric cancer: supplementary analysis of the Japan Clinical Oncology Group study (JCOG0912). Journal of gastroenterology, 58:519-526, 2023
11. Endo M, Kataoka T, Fujiwara T, Tsukushi S, Takahashi M, Kobayashi E, Yamada Y, Tanaka T, Nezu Y, Hiraga H, Wasa J, Nagano A, Nakano K, Nakayama R, Hamada T, Kawano M, Torigoe T, Sakamoto A, Asanuma K, Morii T, Machida R, Sekino Y, Fukuda H, Oda Y, Ozaki T, Tanaka K. Protocol for the 2ND-STEP study, Japan Clinical Oncology Group study JCOG1802: a randomized phase II trial of second-line treatment for advanced soft tissue sarcoma comparing trabectedin, eribulin and pazopanib. BMC cancer, 23:219, 2023
12. Aokage K, Suzuki K, Saji H, Wakabayashi M, Kataoka T, Sekino Y, Fukuda H, Endo M, Hattori A, Mimae T, Miyoshi T, Isaka M, Yoshioka H, Nakajima R, Nakagawa K, Okami J, Ito H, Kuroda H, Tsuboi M, Okumura N, Takahama M, Ohde Y, Aoki T, Tsutani Y, Okada M, Watanabe SI. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. The Lancet. Respiratory medicine, 11:540-549, 2023
13. Nakachi K, Ikeda M, Konishi M, Nomura S, Katayama H, Kataoka T, Todaka A, Yanagimoto H, Morinaga S, Kobayashi S, Shimada K, Takahashi Y, Nakagohri T, Gotoh K, Kamata K, Shimizu Y, Ueno M, Ishii H, Okusaka T, Furuse J. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet (London, England), 401:195-203, 2023
14. Nishikawa T, Hasegawa K, Matsumoto K, Mori M, Hirashima Y, Takehara K, Ariyoshi K, Kato T, Yagishita S, Hamada A, Kawasaki M, Kawashima S, Tomatsuri S, Nagasaka Y, Yoshida H, Machida R, Hirakawa A, Nakamura K, Yonemori K. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. Journal of clinical oncology, 41:2789-2799, 2023
15. Suzuki T, Terada M, Machida R, Kataoka T, Ito Y, Kataoka K, Maruyama D, Nagai H, Lymphoma Study Group of the Japan Clinical Oncology Group. Randomized phase III study of daratumumab versus bortezomib plus daratumumab as maintenance therapy after D-MPB for transplant-ineligible patients with untreated multiple myeloma (JCOG1911, B-DASH study). Japanese Journal of Clinical Oncology, 53:349-354, 2023
16. Yoshino I, Moriya Y, Suzuki K, Wakabayashi M, Saji H, Aokage K, Suzuki M, Ito H, Matsumoto I, Kobayashi M, Okamoto T, Okada M, Yamashita M, Ikeda N, Nakamura S, Kataoka T, Tsuboi M, Watanabe S. Long-term Outcome of Patients with Peripheral Ground Glass Opacity Dominant Lung Cancer after Sublobar Resections. The Journal of Thoracic and Cardiovascular Surgery, 2023
17. Mizusawa J, Tokunaga M, Machida N, Yabusaki H, Kawabata R, Imamura H, Kinoshita T, Nomura T, Nunobe S, Tsuji K, Katayama H, Fukuda H, Boku N, Yoshikawa T, Terashima M, Stomach Cancer Study Group of the Japan Clinical Oncology Group. Protocol digest of a phase III trial to evaluate the efficacy of preoperative chemotherapy with S-1 plus oxaliplatin followed by D2 gastrectomy with postoperative S-1 in locally advanced gastric cancer: Japan Clinical Oncology Group study JCOG1509 (NAGISA Trial). Japanese Journal of Clinical Oncology, 53:168-173, 2022
18. Kiyota N, Tahara M, Mizusawa J, Kodaira T, Fujii H, Yamazaki T, Mitani H, Iwae S, Fujimoto Y, Onozawa Y, Hanai N, Ogawa T, Hara H, Monden N, Shimura E, Minami S, Fujii T, Tanaka K, Homma A, Yoshimoto S, Oridate N, Omori K, Ueda T, Okami K, Ota I, Shiga K, Sugasawa M, Asakage T, Saito Y, Murono S, Nishimura Y, Nakamura K, Hayashi R. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. Journal of clinical oncology, 40:1980-1990, 2022
19. Kobayashi T, Yamamoto K, Kagami Y, Machida R, Miyazaki K, Nakamura S, Kuroda J, Maruyama D, Nagai H. Prognostic value of the Kyoto Prognostic Index in higher-risk diffuse large B-cell lymphomas treated by upfront autologous stem cell transplantation in JCOG0908 trial. Japanese journal of clinical oncology, 52:583-588, 2022
20. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet (London, England), 399:1607-1617, 2022
21. Urakawa H, Nagano A, Machida R, Tanaka K, Kataoka T, Sekino Y, Nishida Y, Takahashi M, Kunisada T, Kawano M, Yoshida Y, Takagi T, Sato K, Hiruma T, Hatano H, Tsukushi S, Sakamoto A, Akisue T, Hiraoka K, Ozaki T. A randomized phase III trial of denosumab before curettage for giant cell tumor of bone. JCOG1610. Japanese journal of clinical oncology, 52:1021-1028, 2022
22. Mitani S, Kato K, Daiko H, Ito Y, Nozaki I, Kojima T, Yano M, Nakagawa S, Ueno M, Watanabe M, Tsunoda S, Abe T, Kadowaki S, Kadota T, Sasaki K, Machida R, Kitagawa Y. Second primary malignancies in patients with clinical T1bN0 esophageal squamous cell carcinoma after definitive therapies: supplementary analysis of the JCOG trial: JCOG0502. Journal of gastroenterology, 57:455-463, 2022
23. Hagi T, Kurokawa Y, Mizusawa J, Fukagawa T, Katai H, Sano T, Misawa K, Fukushima N, Kawachi Y, Sasako M, Yoshikawa T, Terashima M. Impact of tumor-related factors and inter-institutional heterogeneity on preoperative T staging for gastric cancer. Future oncology (London, England), 18:2511-2519, 2022
24. Ohue M, Iwasa S, Mizusawa J, Kanemitsu Y, Shiozawa M, Nishizawa Y, Ueno H, Katsumata K, Yasui M, Tsukamoto S, Katayama H, Fukuda H, Shimada Y. A randomized controlled trial comparing perioperative vs. postoperative mFOLFOX6 for lower rectal cancer with suspected lateral pelvic lymph node metastasis (JCOG1310): a phase II/III randomized controlled trial. Japanese journal of clinical oncology, 52:850-858, 2022
25. Kawakami T, Mizusawa J, Hasegawa H, Imazeki H, Kano K, Sato Y, Iwasa S, Takiguchi S, Kurokawa Y, Doki Y, Boku N, Yoshikawa T, Terashima M. Usefulness of an S-1 dosage formula: an exploratory analysis of randomized clinical trial (JCOG1001). Gastric cancer, 25:1073-1081, 2022
26. Nozaki M, Kagami Y, Takahashi M, Machida R, Sekino Y, Shibata T, Ito Y, Nishimura Y, Teshima T, Ushijima H, Nagata Y, Matsumoto Y, Akimoto T, Takahashi K, Murayama S, Uno T, Tsujino K, Hamamoto Y, Nakagawa K, Kodaira T, Hiraoka M. Evaluation of breast cosmetic changes with a computer-software; the breast cancer conservative treatment cosmetic results (BCCT. core) in hypofractionated whole breast irradiation after breast-conserving surgery-supplementary analysis of multicenter single-arm confirmatory trial: JCOG0906. Breast cancer (Tokyo, Japan), 29:1042-1049, 2022
27. Tanaka K, Machida R, Kawai A, Nakayama R, Tsukushi S, Asanuma K, Matsumoto Y, Hiraga H, Hiraoka K, Watanuki M, Yonemoto T, Abe S, Katagiri H, Nishida Y, Nagano A, Suehara Y, Kawashima H, Kawano M, Morii T, Hatano H, Toguchida J, Okuma T, Takeyama M, Takenaka S, Akisue T, Furuta T, Emori M, Hiruma T, Outani H, Yamamoto T, Kataoka T, Fukuda H, Ozaki T, Iwamoto Y. Perioperative Adriamycin plus ifosfamide vs. gemcitabine plus docetaxel for high-risk soft tissue sarcomas: randomised, phase II/III study JCOG1306. British journal of cancer, 127:1487-1496, 2022
28. Maruyama D, Iida S, Machida R, Kusumoto S, Fukuhara N, Yamauchi N, Miyazaki K, Yoshimitsu M, Kuroda J, Tsukamoto N, Tsujimura H, Usuki K, Yamauchi T, Utsumi T, Mizuno I, Takamatsu Y, Nagata Y, Ota S, Ohtsuka E, Hanamura I, Suzuki Y, Yoshida S, Yamasaki S, Suehiro Y, Kamiyama Y, Fukuhara S, Tsukasaki K, Nagai H. Final analysis of randomized phase II study optimizing melphalan, prednisolone, bortezomib in multiple myeloma (JCOG1105). Cancer science, 113:3267-3270, 2022
29. Takeuchi H, Ito Y, Machida R, Kato K, Onozawa M, Minashi K, Yano T, Nakamura K, Tsushima T, Hara H, Okuno T, Hironaka S, Nozaki I, Ura T, Chin K, Kojima T, Seki S, Sakanaka K, Fukuda H, Kitagawa Y. A Single-Arm Confirmatory Study of Definitive Chemoradiation Therapy Including Salvage Treatment for Clinical Stage II/III Esophageal Squamous Cell Carcinoma (JCOG0909 Study). International journal of radiation oncology, biology, physics, 114:454-462, 2022
30. Kurokawa Y, Doki Y, Mizusawa J, Yoshikawa T, Yamada T, Kimura Y, Takiguchi S, Nishida Y, Fukushima N, Cho H, Kaji M, Hirao M, Sasako M, Terashima M. Five-year follow-up of a randomized clinical trial comparing bursectomy and omentectomy alone for resectable gastric cancer (JCOG1001). The British journal of surgery, 110:50-56, 2022
31. Suzuki T, Maruyama D, Machida R, Kataoka T, Fukushima N, Takayama N, Ohba R, Omachi K, Imaizumi Y, Tokunaga M, Katsuya H, Yoshida I, Sunami K, Kurosawa M, Kubota N, Morimoto H, Kobayashi M, Yamamoto K, Kameoka Y, Kagami Y, Tabayashi T, Maruta M, Kobayashi T, Iida S, Nagai H. Prognostic impact of the UK Myeloma Research Alliance Risk Profile in transplant-ineligible patients with multiple myeloma who received a melphalan, prednisolone, and bortezomib regimen: A supplementary analysis of JCOG1105. Hematological oncology, 2022
32. Aokage K, Tsuboi M, Zenke Y, Horinouchi H, Nakamura N, Ishikura S, Nishikawa H, Kumagai S, Koyama S, Kanato K, Kataoka T, Wakabayashi M, Fukutani M, Fukuda H, Ohe Y, Watanabe SI. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non-small cell lung cancer. Japanese journal of clinical oncology, 52:383-387, 2022
33. Shiraishi Y, Hakozaki T, Nomura S, Kataoka T, Tanaka K, Miura S, Sekino Y, Ando M, Horinouchi H, Ohe Y, Okamoto I. A Multicenter, Randomized Phase III Study Comparing Platinum Combination Chemotherapy Plus Pembrolizumab With Platinum Combination Chemotherapy Plus Nivolumab and Ipilimumab for Treatment-Naive Advanced Non-Small Cell Lung Cancer Without Driver Gene Alterations: JCOG2007 (NIPPON Study). Clinical lung cancer, 23:e285-e288, 2022
34. Nozaki I, Machida R, Kato K, Daiko H, Ito Y, Kojima T, Yano M, Ueno M, Nakagawa S, Kitagawa Y. Long-term survival of patients with T1bN0M0 esophageal cancer after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter trial. Surgical endoscopy, 36:4275-4282, 2022
35. Terashima M, Sano T, Mizusawa J, Umemura K, Tokunaga M, Omori T, Cho H, Hasegawa Y, Akiyama Y, Tsujimoto H, Kawashima Y, Kawachi Y, Lee SW, Kano K, Hasegawa H, Boku N, Yoshikawa T, Sasako M. Prediction of the peritoneal recurrence via the macroscopic diagnosis of the serosal invasion in patients with gastric cancer: Supplementary analysis of JCOG0110. European journal of surgical oncology, 48:1753-1759, 2022
36. Ohue M, Fujita S, Mizusawa J, Kanemitsu Y, Hamaguchi T, Tsukamoto S, Noura S, Yasui M, Itoh M, Shiomi A, Komori K, Watanabe J, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Moriya Y. Preoperative and postoperative prognostic factors of patients with stage II/III lower rectal cancer without neoadjuvant therapy in the clinical trial (JCOG0212). Japanese journal of clinical oncology, 52:114-121, 2022
37. Takii Y, Mizusawa J, Kanemitsu Y, Komori K, Shiozawa M, Ohue M, Ikeda S, Takiguchi N, Kobatake T, Ike H, Sato T, Tomita N, Ota M, Masaki T, Hamaguchi T, Shida D, Katayama H, Shimada Y, Fukuda H. The Conventional Technique Versus the No-touch Isolation Technique for Primary Tumor Resection in Patients With Colon Cancer (JCOG1006): A Multicenter, Open-label, Randomized, Phase III Trial. Annals of surgery, 275:849-855, 2022
38. Oshima K, Kato K, Ito Y, Daiko H, Nozaki I, Nakagawa S, Shibuya Y, Kojima T, Toh Y, Okada M, Hironaka S, Akiyama Y, Komatsu Y, Maejima K, Nakagawa H, Onuki R, Nagai M, Kato M, Kanato K, Kuchiba A, Nakamura K, Kitagawa Y. Prognostic biomarker study in patients with clinical stage I esophageal squamous cell carcinoma: JCOG0502-A1. Cancer science, 113:1018-1027, 2022
39. Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J. Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA oncology, 8:1447-1455, 2022
40. Inada M, Nishimura Y, Ishikura S, Ishikawa K, Murakami N, Kodaira T, Ito Y, Tsuchiya K, Murakami Y, Saito J, Akimoto T, Nakata K, Yoshimura M, Teshima T, Toshiyasu T, Ota Y, Minemura T, Shimizu H, Hiraoka M. Organs-at-risk dose constraints in head and neck intensity-modulated radiation therapy using a dataset from a multi-institutional clinical trial (JCOG1015A1). Radiation oncology (London, England), 17:133, 2022
41. Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, Yoshino I, Tsuboi M, Nakamura S, Nakamura K, Mitsudomi T, Asamura H. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. The Journal of thoracic and cardiovascular surgery, 163:289-301.e2, 2022
42. Hironaka S, Sadachi R, Machida N, Iwasa S, Yamada Y, Sasako M, Yoshikawa T, Boku N, Terashima M. Association of renal function with the safety and efficacy of cisplatin plus S-1 therapy and docetaxel plus cisplatin plus S-1 therapy in patients with advanced gastric cancer: an exploratory analysis of JCOG1013. Japanese journal of clinical oncology, 52:14-23, 2022
43. Misawa K, Kurokawa Y, Mizusawa J, Takiguchi S, Doki Y, Makino S, Choda Y, Takeno A, Tokunaga M, Sano T, Sasako M, Yoshikawa T, Terashima M. Negative impact of intraoperative blood loss on long-term outcome after curative gastrectomy for advanced gastric cancer: exploratory analysis of the JCOG1001 phase III trial. Gastric cancer, 25:459-467, 2022
44. Yoshida M, Takizawa K, Hasuike N, Ono H, Boku N, Kadota T, Mizusawa J, Oda I, Yoshida N, Horiuchi Y, Hirasawa K, Morita Y, Yamamoto Y, Muto M. Second gastric cancer after curative endoscopic resection of differentiated-type early gastric cancer: post-hoc analysis of a single-arm confirmatory trial. Gastrointestinal endoscopy, 95:650-659, 2022
45. Yamada I, Morizane C, Okusaka T, Mizusawa J, Kataoka T, Ueno M, Ikeda M, Okano N, Todaka A, Shimizu S, Mizuno N, Sekimoto M, Tobimatsu K, Yamaguchi H, Nishina T, Shirakawa H, Kojima Y, Oono T, Kawamoto Y, Furukawa M, Iwai T, Sudo K, Okamura K, Yamashita T, Kato N, Shioji K, Shimizu K, Nakagohri T, Kamata K, Ishii H, Furuse J. The clinical outcomes of combination chemotherapy in elderly patients with advanced biliary tract cancer: an exploratory analysis of JCOG1113. Scientific reports, 12:987, 2022
46. Oda Y, Tanaka K, Hirose T, Hasegawa T, Hiruta N, Hisaoka M, Yoshimoto M, Otsuka H, Bekki H, Ishii T, Endo M, Kunisada T, Hiruma T, Tsuchiya H, Katagiri H, Matsumoto Y, Kawai A, Nakayama R, Kawashima H, Takenaka S, Emori M, Watanuki M, Yoshida Y, Okamoto T, Mizusawa J, Fukuda H, Ozaki T, Iwamoto Y, Nojima T. Standardization of evaluation method and prognostic significance of histological response to preoperative chemotherapy in high-grade non-round cell soft tissue sarcomas. BMC cancer, 22:94, 2022
47. Horiuchi Y, Takizawa K, Yoshio T, Mizusawa J, Ono H, Hasuike N, Yano T, Yoshida N, Miwa H, Boku N, Terashima M, Muto M. Pretreatment risk factors for endoscopic noncurative resection of gastric cancers with undifferentiated-type components. Journal of gastroenterology and hepatology, 37:758-765, 2022
48. Yoshio T, Minashi K, Mizusawa J, Morita Y, Tajika M, Fujiwara J, Yamamoto Y, Katada C, Hori S, Yano T, Takizawa K, Fukuda H, Muto M. Effect of chemoradiation on the development of second primary cancers after endoscopic resection of T1 esophageal squamous cell carcinoma. Esophagus, 19:469-476, 2022
