Annual Report 2022
Center for Cancer Registries
Takahiro Higashi, Naoyuki Sato, Yoichiro Tsukada, Manami Fujishita, Jun Umezawa, Mariko Niino, Naoki Sakakibara, Taisuke Ishii, Mami Ueda, Yoshiko Emori, Saya Maruyama, Seiya Kondo, Mika Maeda, Shiho Matsuura, Yuka Takahashi, Saori Uenishi, Mika Mizuochi, Kayoko Kobayashi, Emiko Enjoji, Naoko Takahashi, Mari Watabe, Nanayo Shindo, Eri Yano and Chikako Kamiyama
Introduction
The Center for Cancer Registries is in charge of operating public cancer registries to provide the public with accurate cancer incidence and survival statistics. The Center operates population-based cancer registries (the National Cancer Registry) and hospital-based cancer registries according to new legislation, the Cancer Registry Promotion Act.
The Team and What We Do
1. Population-Based Cancer Registries
The Cancer Registry Promotion Act of 2013 created a new structure called the National Cancer Registry, which mandates that all hospitals in Japan submit basic data to prefectures when they diagnose new patients with cancer. The Center developed an online National Cancer Registry System (NCRS), through which we collect this information from the 47 prefectures. The system started in 2016, and we reported national incidence rates in 2019 to the Ministry of Health, Labour and Welfare (MHLW). In March 2022, the NCRS was updated and moved to a cloud environment to improve the processing performance and operability of the system. In addition, in order to reduce duplication of registration work at hospitals, notification using a common system was started in the two cancer registries in July 2022. For providing cancer registration information, we have introduced an electronic application system to promote utilization and streamline operations. We also started a service for data use for research purposes in 2018, and data use was permitted for 24 studies in 2022.
Educational trainings for cancer registrars and administrative officers of prefectures in charge of cancer control was held in May and December. The online training was attended by 49 and 118 participants, respectively, and the e-learning by 145 and 119 participants, respectively. We closely communicate with the prefectures and continuously strive to improve our cancer surveillance system in Japan. The Population-Based Cancer Registry Database System (PBCRDS), owned by the Center, enables the prefectures to maintain cancer registry data for past years and to link them with the current data in the NCRS. Forty-five registries have introduced the PBCRDS as of March 2023. An external audit on information security in cancer registration was performed by commissioning the cancer registry professional organization. The Center distributes a simplified cancer registry system, “Hos-CanR Lite” to hospitals that submit dates only to the National Cancer Registry.
2. Hospital-Based Cancer Registries
The Hospital-Based Cancer Registry (HBCR) has become a key component in evaluating cancer care in hospitals and has also provided a data frame for population-based registries. Once submitted directly to the Center, the HBCR data help form a national database of hospital-based cancer registries and generate national cancer statistics geared more towards clinical aspects than population-based cancer registries. The Cancer Registry Promotion Act encourages hospitals specialized in cancer care or those playing key roles in the region to operate the HBCR in accordance with the National Guidelines. The National Guidelines state that the Center should set the standards for the HBCR nationwide and produce the national statistics for cancer care so that the patients can choose hospitals and the government can enhance cancer control activities. The HBCR has been performed at 453 designated cancer care hospitals (DCCHs) and 417 others, including six designated hospitals for pediatric cancer. Individual records for 1,099,864 cancer cases diagnosed in 2021 were collected from 870 hospitals.
To ensure data quality, we organized a series of education programs for cancer registrars. Approximately two hours of e-learning training were conducted for those certified as beginner or intermediate cancer registrars, and 1,063 and 924 individuals participated in the training sessions, respectively. A total of 560 individuals took the renewal examination for beginners, with 475 passing, and 371 individuals took the renewal examination for the intermediate level, with 337 passing.
In addition, freely viewable e-learning videos were provided to those intending to obtain the beginner certification in cancer registration practice, and 618 individuals were certified through the beginner certification exam. In addition, an 18-hour e-learning training course was provided for those seeking intermediate-level certification, and 166 were certified through the intermediate-level certification examination.
Furthermore, an online training program for data analysis for cancer registrars was held.
Table 1. Cancer Patient Data in Hospital-Based Cancer Registries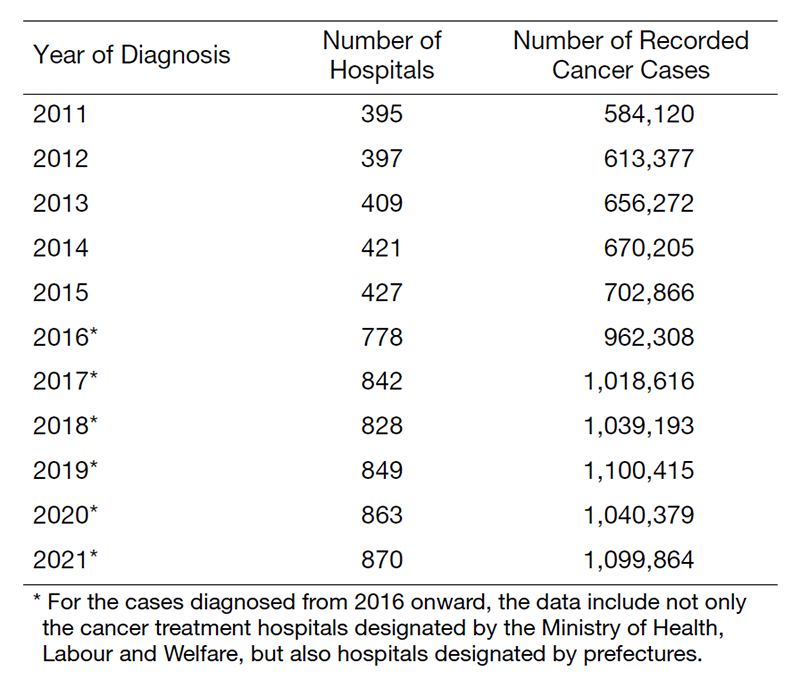

Research Activities
The incidence data from 2019 were published based on the National Cancer Registry, which covers all 47 prefectures, and were analyzed in detail according to cancer site and age group. The results were published on the website in a national report, "Cancer Statistics of Japan".
Hospital-based cancer registries are compiled at the national level in the Center. To improve the quality of cancer care nationwide, we produced an annual report for cases diagnosed in 2020. In addition, we analyzed the actual number of cases in the national cancer registry during the novel coronavirus epidemic and published the results.
The national database was used extensively in research activities both within and outside the Center, while the Secondary Data Use Review Committee approved two proposals for data use.
Education
Prefectural cancer registry personnel and hospital-based cancer registrars were trained as mentioned above; in particular, the Center collaborated closely with the NCC hospitals to educate intermediate-level cancer registrars.
Future Prospects
We plan activities to improve the operation of the National Cancer Registry (NCR) on an ongoing basis. Working with the National Cancer Center’s International Affairs Section, the Center is building a relationship with the International Agency of Research on Cancer (IARC) and other international institutions based on bi-institutional memoranda of understanding.
The hospital-based cancer registries continue to evolve and close monitoring of the care provided to cancer patients in Japan enables better access to quality care. To enable cancer patients and their families to efficiently access the hospital information they need, an interactive website was launched showing the number of cases involved in each patient’s treatment. The data are used for all aspects of cancer control activities, including hospital designation, organization of a care structure for rare cancer patients and quality monitoring of care.
List of papers published in 2022
Journal
1. Omura G, Yoshimoto S, Rikitake R, Eguchi K, Nakamizo M, Nibu KI. Comparison of survival outcomes between adolescent and young adults and older adults with tongue squamous cell carcinoma: a nationwide database study using the head and neck cancer registry of Japan. International journal of clinical oncology, 28:221-228, 2023
2. Hayakawa M, Watanabe O, Shiga K, Fujishita M, Yamaki C, Ogo Y, Takahashi T, Ikeguchi Y, Takayama T. Exploring types of conversational agents for resolving cancer patients’ questions and concerns: Analysis of 100 telephone consultations on breast cancer. Patient education and counseling, 106:75-84, 2023
3. Ishii T, Watanabe T, Higashi T. Baseline cardiac function checkup in patients with gastric or breast cancer receiving trastuzumab or anthracyclines. Cancer medicine, 12:122-130, 2023
4. Ishii T, Watanabe T, Higashi T. Differences in the performance of adjuvant chemotherapy between hemodialysis and nonhemodialysis patients. Cancer medicine, 12:4033-4041, 2023
5. Watanabe T, Rikitake R, Kakuwa T, Ichinose Y, Niino M, Mizushima Y, Ota M, Fujishita M, Tsukada Y, Higashi T. Time to Treatment Initiation for Six Cancer Types: An Analysis of Data from a Nationwide Registry in Japan. World journal of surgery, 47:877-886, 2023
6. Yamamoto S, Sakakibara N, Hirano H, Morizane C, Honma Y, Hijioka S, Okusaka T, Higashi T, Kawai A. The real-world selection of first-line systemic therapy regimen for metastatic gastroenteropancreatic neuroendocrine neoplasm in Japan. Scientific reports, 12:17601, 2022
7. Satake T, Morizane C, Rikitake R, Higashi T, Okusaka T, Kawai A. The epidemiology of rare types of hepatobiliary and pancreatic cancer from national cancer registry. Journal of gastroenterology, 57:890-901, 2022
8. Kanehara R, Goto A, Watanabe T, Inoue K, Taguri M, Kobayashi S, Imai K, Saito E, Katanoda K, Iwasaki M, Ohashi K, Noda M, Higashi T. Association between diabetes and adjuvant chemotherapy implementation in patients with stage III colorectal cancer. Journal of diabetes investigation, 13:1771-1778, 2022
9. Okuyama A, Watabe M, Makoshi R, Takahashi H, Tsukada Y, Higashi T. Impact of the COVID-19 pandemic on the diagnosis of cancer in Japan: analysis of hospital-based cancer registries. Japanese journal of clinical oncology, 52:1215-1224, 2022
10. Ishii T, Nakano E, Watanabe T, Higashi T. Cardiac Function Checkup During Trastuzumab Therapy Among Patients With Breast Cancer. Clinical breast cancer, 22:491-498, 2022
11. Higashi T. Cancer epidemiology and treatment patterns for older persons in Japan: a review of nationwide data and statistics. Japanese journal of clinical oncology, 52:303-312, 2022
12. Ren N, Ogata S, Kiyoshige E, Nishimura K, Nishimura A, Matsuo R, Kitazono T, Higashi T, Ogasawara K, Iihara K. Associations Between Adherence to Evidence-Based, Stroke Quality Indicators and Outcomes of Acute Reperfusion Therapy. Stroke, 53:3359-3368, 2022
13. Ishii T, Mimura I, Nagaoka K, Naito A, Sugasawa T, Kuroda R, Yamada D, Kanki Y, Kume H, Ushiku T, Kakimi K, Tanaka T, Nangaku M. Effect of M2-like macrophages of the injured-kidney cortex on kidney cancer progression. Cell death discovery, 8:480, 2022
14. Ichinose Y, Yang YH, Tsai HJ, Huang RY, Higashi T, Nishida T, Chen LT. Imatinib use for gastrointestinal stromal tumors among older patients in Japan and Taiwan. Scientific reports, 12:22492, 2022
