Annual Report 2023
Department of Head and Neck, Esophageal Medical Oncology
Ken Kato, Yoshitaka Honma, Shun Yamamoto, Kazuki Yokoyama, Kazuhiro Shiraishi, Akihiro Ohara, Mai Itoyama
Introduction
The Department of Head and Neck, Esophageal Medical Oncology focuses on the development of new drugs and establishment of standard chemotherapy regimens, including multimodality treatment with surgery and/or radiotherapy for advanced head and neck cancers (HNCs), consisting of malignancies arising from the oral cavity, nasopharynx, oropharynx, hypopharynx, larynx, nasal/paranasal cavity, salivary gland, ear canal, and thyroid, etc. We also focus on the multimodality treatment of esophageal cancer (EC), mainly chemotherapy and chemoradiotherapy. The main histology of HNC and EC is squamous cell carcinoma. However, there is still a wide variety of histological types, especially in the nasal/paranasal cavity, salivary glands, and gastroesophageal-junctional cancer. Therefore, the pathological diagnosis is essential, making a treatment strategy based on pathological findings significant in advanced HNCs and ECs.
The Team and What We Do
In the fiscal year 2023, the number of patients referred for initial consultation was 223. Including patients referred from other departments for perioperative chemotherapy, induction chemotherapy, chemoradiotherapy, palliative chemotherapy, or consultations, 510 patients were treated, of which 452 patients received pharmacotherapy. The breakdown included 234 patients with esophageal cancer/esophagogastric junction cancer, 7 with esophageal neuroendocrine tumors, 40 with hypopharyngeal cancer, 31 with oropharyngeal cancer, 42 with oral cavity cancer, 10 with laryngeal cancer, 8 with nasopharyngeal cancer, 16 with thyroid cancer, and 64 with other rare esophageal and head and neck cancers. We have also received referrals from other institutions for rare head and neck cancers, such as undifferentiated thyroid cancer and salivary gland cancer.
Research Activities
We have conducted numerous corporate, physician-initiated, and multi-center clinical trials. As the lead author, we have presented at scientific conferences and authored papers. In the fiscal year 2023, a total of 41 patients were registered for clinical trials/studies (Table 1). We presented a total of 81 conference presentations (including co-authorships), of which 46 were presented at international conferences. We also published 32 papers (including co-authored ones), of which 29 were original articles in English and 3 were English reviews. Among these, our department served as the lead or corresponding author in 10 papers. We maintain a list of clinical trials/studies conducted by our department, along with the number of registrations (Table 1).
Table 1. Clinical trials and the number of registered patients.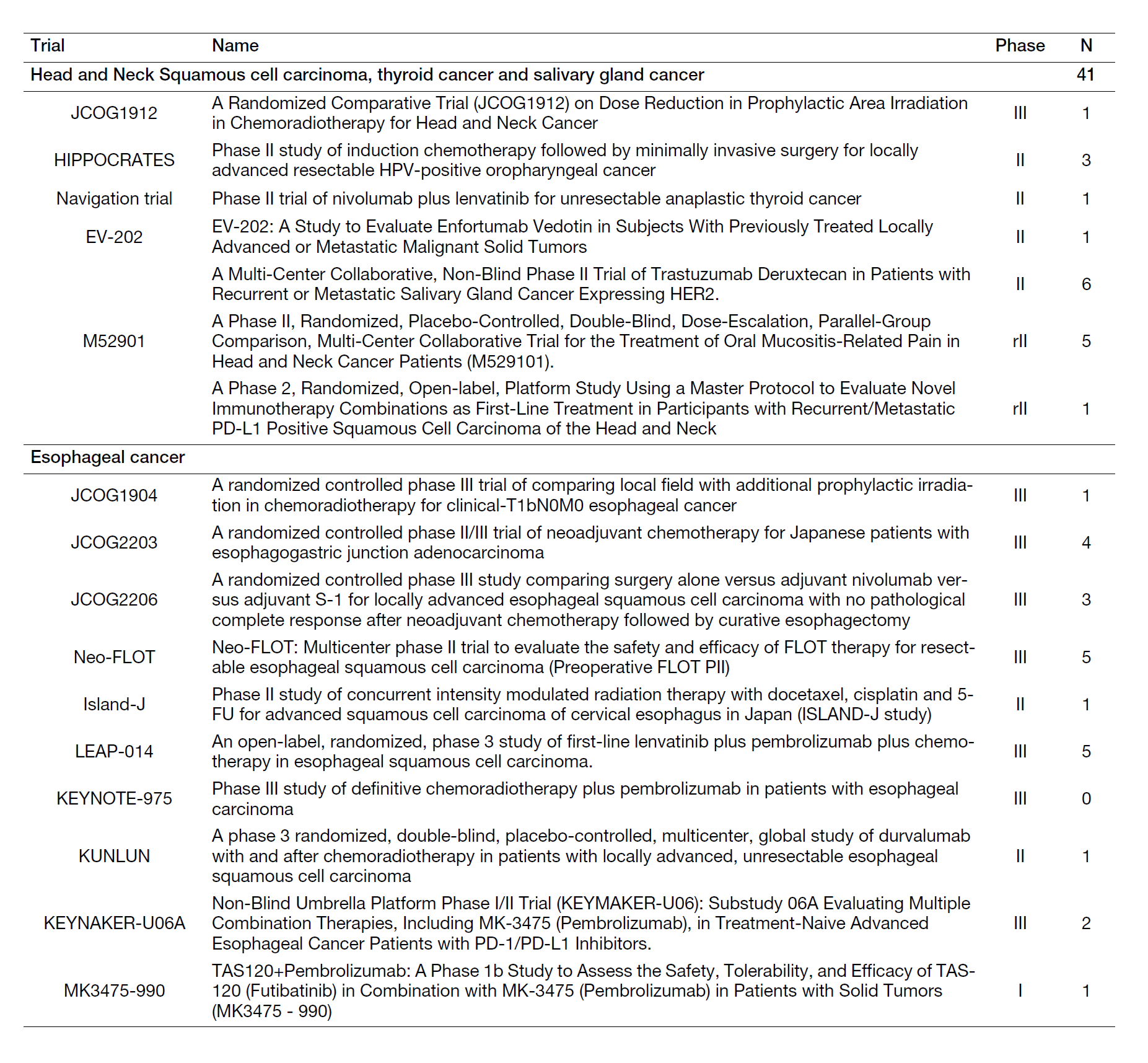

Clinical Trials
With the aim of establishing effective treatments, we are actively conducting clinical research. Our staff plays a central role in proposing and participating in new trials initiated by the Japan Clinical Oncology Group (JCOG), and our institution actively registers patients, contributing to the establishment of new standard treatments. We not only engage in late-stage treatment development but also participate in domestic and international clinical trials from the planning stages to phases I-III for drug development. In addition, we coordinate clinical trials led by physicians for the preoperative treatment of esophageal cancer and register numerous cases as a participating institution for physician-led trials on salivary gland cancer. We are actively involved in translational research (TR) in collaboration with research institutes and other facilities, proposing new physician-led trials to pharmaceutical companies.
Education
As residents of the Department of Head and Neck, Esophageal Medical Oncology, Dr. Akihiro Ohara and Dr. Mai Itoyama, originally from other university otolaryngology departments, have been studying comprehensive treatments for head and neck cancer and esophageal cancer at our institution, expanding the entry of non-internal medicine physicians into the field of medical oncology. In addition, as the first chief resident of the Department of Head and Neck, Esophageal Medical Oncology, Kazuhiro Shiraishi, a medical oncologist, is intensively studying multidisciplinary treatment including pharmacotherapy for esophageal cancer, and is receiving education from both the medical and research aspects to become an opinion leader in the specialty area. We conduct daily rounds and provide education through conferences including residents and staff members. Each staff member provides lectures to residents every three months, and we also distribute them as Zoom or video archives to institutions with former residents and related facilities. We regularly conduct exchange activities with institutions such as Juntendo University Department of Gastroenterology and Nagasaki University Otolaryngology, using Zoom for case conferences and lectures. We are actively attempting to discover new talents. Resident-led presentations at conferences included 18 presentations (9 domestic and 9 international) and 11 English articles.
Future Prospects
The treatment of head and neck cancer and esophageal cancer has not yet reached many regions, therefore we collaborate with the Regional Medical Coordination Office to increase referrals to our hospital. While we base our treatments on standard practices, we actively strive for continuous improvement to meet the unmet needs of patients, aiming to establish a brand that makes other hospitals want to refer patients to us. On the research front, we actively engage with global teams from pharmaceutical companies at conferences and propose research ideas, highlighting the strengths of our hospital, which boasts one of the world's largest patient populations. We will continue to propose physician-initiated trials and conduct multinational clinical trials in Asia. Collaborating with the research institute and our hospital's Clinical Development Promotion Division, we will focus on translational research (TR) to develop new treatments. In terms of education, through resident education, we aim to train not only highly specialized professionals in cancer drug therapy but also young oncologists with research-oriented minds who are well-versed in comprehensive treatments, including surgery and radiation therapy. We aspire to nurture the next generation of researchers.
List of papers published in 2023
Journal
1. Watanabe T, Honma Y, Yonemori K, Sunami K, Yoshimoto S, Mori T. High-grade intraductal carcinoma of the parotid gland harboring CTNNA1::ALK rearrangement: Changes in genetic status using genetic testing during treatment with an ALK inhibitor. Head & neck, 46:E26-E31, 2024
2. Hirose T, Yamamoto S, Honma Y, Yokoyama K, Hirano H, Okita N, Shoji H, Iwasa S, Takashima A, Ishiyama K, Oguma J, Daiko H, Maeda S, Kato K. Preoperative docetaxel, cisplatin, and 5-fluorouracil for resectable locally advanced esophageal and esophagogastric junctional adenocarcinoma. Esophagus, 2024
3. Homma A, Ando M, Hanai N, Harada H, Honma Y, Kanda T, Kano S, Kawakita D, Kiyota N, Kizawa Y, Nakagawa M, Ogawa T, Shinomiya H, Shinozaki T, Suzuki M, Tsuji T, Yasuda K, Zenda S, Kodaira T, Kirita T, Nibu KI. Summary of Japanese clinical practice guidelines for head and neck cancer - 2022 update edited by the Japan society for head and neck cancer. Auris, nasus, larynx, 51:174-188, 2024
4. Yoshinami Y, Nishimura E, Hosokai T, Yamamoto S, Matsuda S, Nomura M, Kawakubo H, Kato K, Kitagawa Y. Rare malignant neoplasm of the esophagus: current status and future perspectives. Japanese journal of clinical oncology, 54:111-120, 2024
5. Ikeda G, Miyakoshi J, Yamamoto S, Kato K. Nivolumab in unresectable advanced, recurrent or metastatic esophageal squamous cell carcinoma. Future oncology (London, England), 20:665-677, 2024
6. Kitagawa Y, Matsuda S, Gotoda T, Kato K, Wijnhoven B, Lordick F, Bhandari P, Kawakubo H, Kodera Y, Terashima M, Muro K, Takeuchi H, Mansfield PF, Kurokawa Y, So J, Mönig SP, Shitara K, Rha SY, Janjigian Y, Takahari D, Chau I, Sharma P, Ji J, de Manzoni G, Nilsson M, Kassab P, Hofstetter WL, Smyth EC, Lorenzen S, Doki Y, Law S, Oh DY, Ho KY, Koike T, Shen L, van Hillegersberg R, Kawakami H, Xu RH, Wainberg Z, Yahagi N, Lee YY, Singh R, Ryu MH, Ishihara R, Xiao Z, Kusano C, Grabsch HI, Hara H, Mukaisho KI, Makino T, Kanda M, Booka E, Suzuki S, Hatta W, Kato M, Maekawa A, Kawazoe A, Yamamoto S, Nakayama I, Narita Y, Yang HK, Yoshida M, Sano T. Clinical practice guidelines for esophagogastric junction cancer: Upper GI Oncology Summit 2023. Gastric cancer, 27:401-425, 2024
7. Yada M, Yamamoto S, Honma Y, Hirano H, Okita N, Shoji H, Iwasa S, Takashima A, Nagahara A, Kato K. Retrospective Analysis of Definitive Chemoradiotherapy With FOLFOX in Patients With Esophageal Cancer Intolerant to Cisplatin. In vivo (Athens, Greece), 38:761-766, 2024
8. Kita R, Matsuda S, Nomura M, Machida R, Sasaki K, Kato K, Goto R, Yoshioka T, Yamamoto S, Tsushima T, Fukuda H, Takeuchi H, Kitagawa Y, Japan Esophageal Oncology Group of the Japan Clinical Oncology Group. Protocol digest of a randomized controlled Phase III study comparing surgery alone versus adjuvant nivolumab versus adjuvant S-1 for locally advanced oesophageal squamous cell carcinoma with no pathological complete response after neoadjuvant chemotherapy followed by curative esophagectomy: Japan Clinical Oncology Group study JCOG2206 (SUNRISE Trial). Kita R, Matsuda S, Nomura M, Machida R, Sasaki K, Kato K, Goto R, Yoshioka T, Yamamoto S, Tsushima T, Fukuda H, Takeuchi H, Kitagawa Y, Japan Esophageal Oncology Group of the Japan Clinical Oncology Group, 54:212–216, 2024
9. Nakamura Y, Yamashita R, Okamoto W, Komatsu Y, Yuki S, Ueno M, Kato K, Taniguchi H, Kagawa Y, Denda T, Hara H, Esaki T, Moriwaki T, Sunakawa Y, Oki E, Nagashima F, Nishina T, Satoh T, Kawakami H, Yamaguchi K, Ohtsubo K, Kato T, Horita Y, Tsuji A, Yasui H, Goto M, Hamamoto Y, Wakabayashi M, Ikeno T, Shitara K, Bando H, Tsuchihara K, Miki I, Ichiki H, Ohtsu A, Yoshino T. Efficacy of Targeted Trials and Signaling Pathway Landscape in Advanced Gastrointestinal Cancers From SCRUM-Japan GI-SCREEN: A Nationwide Genomic Profiling Program. JCO precision oncology, 7:e2200653, 2023
10. Eguchi K, Kobayashi K, Honma Y, Ryo E, Sakyo A, Yokoyama K, Watanabe T, Aihara Y, Sakai A, Matsumoto Y, Sakai T, Omura G, Yatabe Y, Yoshimoto S, Mori T. Clinical and pathological features of second primary neoplasms arising in head and neck reconstructive skin flaps. Scientific reports, 13:11214, 2023
11. Eguchi K, Omura G, Murakami N, Honma Y, Yokoyama K, Watanabe T, Aihara Y, Sakai A, Matsumoto Y, Sakai T, Kobayashi K, Igaki H, Yoshimoto S. Comparison of Survival Outcomes Between Larynx-Preserving Open Partial Pharyngectomy and Radiotherapy or Chemoradiotherapy in Patients with Hypopharyngeal Squamous Cell Carcinoma: A Single-Center Retrospective Analysis with Inverse Probability of Treatment Weighting Adjustments. Annals of surgical oncology, 30:6867-6874, 2023
12. Matsuzaki J, Kato K, Oono K, Tsuchiya N, Sudo K, Shimomura A, Tamura K, Shiino S, Kinoshita T, Daiko H, Wada T, Katai H, Ochiai H, Kanemitsu Y, Takamaru H, Abe S, Saito Y, Boku N, Kondo S, Ueno H, Okusaka T, Shimada K, Ohe Y, Asakura K, Yoshida Y, Watanabe SI, Asano N, Kawai A, Ohno M, Narita Y, Ishikawa M, Kato T, Fujimoto H, Niida S, Sakamoto H, Takizawa S, Akiba T, Okanohara D, Shiraishi K, Kohno T, Takeshita F, Nakagama H, Ota N, Ochiya T. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI cancer spectrum, 7:pkac080, 2023
13. Kato K, Doki Y, Ogata T, Motoyama S, Kawakami H, Ueno M, Kojima T, Shirakawa Y, Okada M, Ishihara R, Kubota Y, Amaya-Chanaga C, Chen T, Matsumura Y, Kitagawa Y. First-line nivolumab plus ipilimumab or chemotherapy versus chemotherapy alone in advanced esophageal squamous cell carcinoma: a Japanese subgroup analysis of open-label, phase 3 trial (CheckMate 648/ONO-4538-50). Esophagus, 20:291-301, 2023
14. Kadono T, Iwasa S, Nagashima K, Oshima K, Yamamoto S, Hirano H, Okita N, Shoji H, Honma Y, Takashima A, Kato K, Ushijima T, Boku N. Progression patterns and site-specific responses in advanced gastric cancer patients treated with nivolumab. Cancer medicine, 12:9322-9331, 2023
15. Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, Park SR, Ping L, Jiang Y, Zhang J, Wu X, Yao Y, Shen L, Kojima T, Gotovkin E, Ishihara R, Wyrwicz L, Van Cutsem E, Jimenez-Fonseca P, Lin CY, Wang L, Shi J, Li L, Yoon HH. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. The Lancet. Oncology, 24:483-495, 2023
16. Pape M, Vissers PAJ, Kato K, Haj Mohammad N, Klarenbeek B, van Laarhoven HWM, Matsuda T, Verhoeven RHA. A population-based comparison of patients with metastatic esophagogastric carcinoma between Japan and the Netherlands. Journal of cancer research and clinical oncology, 149:13323-13330, 2023
17. Lee DH, Kim HR, Keam B, Kato K, Kuboki Y, Gao H, Yovine A, Robbins SH, Ahn MJ. Safety and tolerability of first-line durvalumab with tremelimumab and chemotherapy in esophageal squamous cell carcinoma. Cancer medicine, 12:16066-16075, 2023
18. Ando K, Nakamura Y, Kitao H, Shimokawa M, Kotani D, Bando H, Nishina T, Yamada T, Yuki S, Narita Y, Hara H, Ohta T, Esaki T, Hamamoto Y, Kato K, Yamamoto Y, Minashi K, Ohtsubo K, Izawa N, Kawakami H, Kato T, Satoh T, Okano N, Tsuji A, Yamazaki K, Yoshino T, Maehara Y, Oki E. Mutational spectrum of TP53 gene correlates with nivolumab treatment efficacy in advanced gastric cancer (TP53MUT study). British journal of cancer, 129:1032-1039, 2023
19. Baba T, Kusumoto M, Kato T, Kurihara Y, Sasaki S, Oikado K, Saito Y, Endo M, Fujiwara Y, Kenmotsu H, Sata M, Takano T, Kato K, Hirata K, Katagiri T, Saito H, Kuwano K. Clinical and imaging features of interstitial lung disease in cancer patients treated with trastuzumab deruxtecan. International journal of clinical oncology, 28:1585-1596, 2023
20. Okumura T, Fujii T, Terabayashi K, Kojima T, Takeda S, Kashiwada T, Toriyama K, Hijioka S, Miyazaki T, Yamamoto M, Tanabe S, Shirakawa Y, Furukawa M, Honma Y, Hoshino I, Nabeya Y, Yamaguchi H, Uemoto S, Shimada Y, Matsubara H, Ozawa S, Makuuchi H, Imamura M. MicroRNAs associated with postoperative outcomes in patients with limited stage neuroendocrine carcinoma of the esophagus. Oncology letters, 26:276, 2023
21. Honma Y, Ikeda M, Hijioka S, Matsumoto S, Ito T, Aoki T, Furuse J. Optimal first-line treatment strategies of systemic therapy for unresectable gastrointestinal neuroendocrine tumors based on the opinions of Japanese experts. Investigational new drugs, 41:777-786, 2023
22. Itoyama M, Ohara A, Yokoyama K, Yamamoto S, Kato K, Mori T, Igaki H, Nakano E, Yamazaki N, Sunami K, Nishigori C, Honma Y. Successful Use of Anti-PD-1 Antibody to Treat Multiple Metastatic Carcinomas in a Patient with Xeroderma Pigmentosum: Case Report and Literature Review. Clinical Images and Case Reports Journal, 5:312, 2023
23. Kadono T, Yamamoto S, Hirose T, Ikeda G, Ohara A, Itoyama M, Yokoyama K, Honma Y, Hashimoto T, Sekine S, Ishiyama K, Oguma J, Daiko H, Kato K. Safety and short-term efficacy of preoperative FOLFOX therapy in patients with resectable esophageal squamous cell carcinoma who are ineligible for cisplatin. Esophagus, 20:109-115, 2023
24. Ura T, Hironaka S, Tsubosa Y, Mizusawa J, Kato K, Tsushima T, Fushiki K, Chin K, Tomori A, Okuno T, Matsushita H, Kojima T, Doki Y, Kusaba H, Fujitani K, Seki S, Kitagawa Y. Early tumor shrinkage and depth of response in patients with metastatic esophageal cancer treated with 2-weekly docetaxel combined with cisplatin plus fluorouracil: an exploratory analysis of the JCOG0807. Esophagus, 20:272-280, 2023
25. Chin K, Yamamoto S, Takahashi M, Kadowaki S, Kubota Y, Amanuma Y, Okada M, Kanda M, Kimura Y, Nogi Y, Arimitsu Y, Kitagawa Y. Effectiveness of taxanes following nivolumab in patients with advanced esophageal squamous cell carcinoma: a retrospective chart review of patients in ATTRACTION-3. Esophagus, 20:302-308, 2023
26. Oguma J, Ishiyama K, Kurita D, Kanematsu K, Kubo K, Utsunomiya D, Yamamoto S, Honma Y, Kato K, Daiko H. Significance of lymphovascular invasion in esophageal squamous cell carcinoma undergoing neoadjuvant chemotherapy followed by esophagectomy. Esophagus, 20:215-224, 2023
27. Kitagawa Y, Ishihara R, Ishikawa H, Ito Y, Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, Kuribayashi S, Kono K, Kojima T, Takeuchi H, Tsushima T, Toh Y, Nemoto K, Booka E, Makino T, Matsuda S, Matsubara H, Mano M, Minashi K, Miyazaki T, Muto M, Yamaji T, Yamatsuji T, Yoshida M. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: part 2. Esophagus, 20:373-389, 2023
28. Kim Y, Yamamoto S, Kato K. Profile of Nivolumab in the Treatment of Resected Esophageal Squamous Cell Carcinoma: A Review of the Clinical Data. Cancer management and research, 15:399-406, 2023
29. Hirai H, Nakaguro M, Tada Y, Saigusa N, Kawakita D, Honma Y, Kano S, Tsukahara K, Ozawa H, Okada T, Okami K, Yamazaki K, Sato Y, Urano M, Kajiwara M, Utsumi Y, Shimura T, Fushimi C, Shimizu A, Kondo T, Imanishi Y, Sakai A, Sato Y, Togashi T, Hanazawa T, Matsuki T, Yamazaki K, Nagao T. Prognostic value and clinicopathological roles of the tumor immune microenvironment in salivary duct carcinoma. Virchows Archiv, 483:367-379, 2023
30. Shiraishi K, Yamamoto S, Kato K. Combination immunotherapy in chemotherapy in gastric cancer. The Lancet. Oncology, 24:1054-1055, 2023
31. Mishima S, Nakamura Y, Tukachinsky H, Taniguchi H, Kadowaki S, Kato K, Oki E, Satoh T, Aoki D, Yamazaki K, Esaki T, Ueno M, Nishina T, Sunakawa Y, Denda T, Bando H, Naomi K, Horasawa S, Abutani H, Lee J, Madison R, Oxnard G, Yoshino T. Validity and utility of blood tumor mutational burden (bTMB) is dependent on circulating tumor DNA (ctDNA) shed: SCRUM-Japan MONSTAR-SCREEN. Journal of Liquid Biopsy, 1:100003, 2023
32. Tsunoda S, Tsubosa Y, Sasaki K, Machida R, Kita R, Fukuda H, Koyanagi K, Takeuchi H, Kamei T, Mine S, Noma K, Ken K, Kitagawa Y, the Japan Esophageal Oncology Group of Japan Clinical Oncology Group. A multicenter randomized controlled trial of esophagectomy with or without prophylactic supraclavicular node dissection: a phase 3 trial (JCOG2013, MODERN3). Japanese Journal of Clinical Oncology, 53:858–862, 2023
