Home > Division of Molecular Pharmacology
Division of Molecular Pharmacology
Our Mission
Our laboratory aims for implementing anticancer drug discovery & development in JAPAN. In this regard, we are working on “Clinical pharmacological research at a molecular level” by using next generation PK/PD/PGx analyses, novel Molecular Drug Imaging system, and J-PDX library.
Clinical Pharmacology
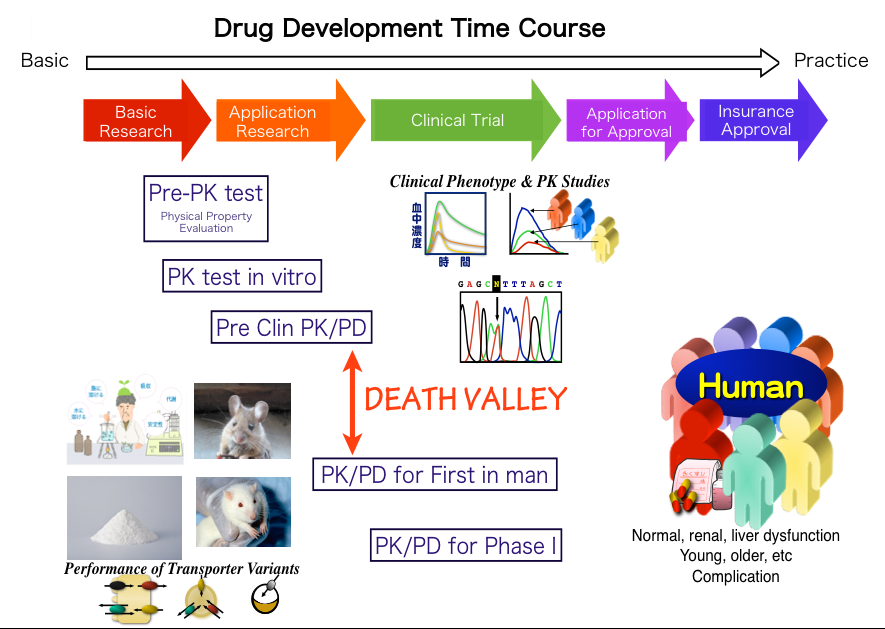
Clinical pharmacology is the science of drugs and their clinical use. Clinical pharmacology connects the gap between medical practice and laboratory science especially in the development of anticancer drugs. In phase I clinical trials of anticancer drugs, clinical pharmacology research includes various kind of analyses such as pharmacokinetics (PK), pharmacodynamics (PD), pharmacogenomics (PGx), toxicology, drug-drug interaction, transporter, and so on. These clinical pharmacological analyses are indispensable for connecting the “Death Valley” between pre-clinical and clinical trials.
PK/PD/PGx project
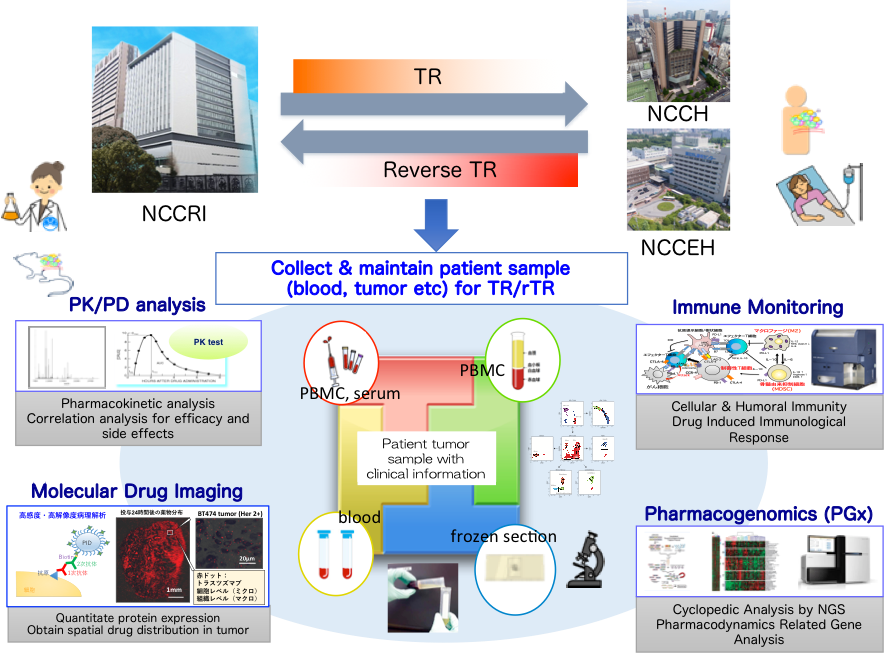
In recent years, anticancer drug development shift from conventional cytotoxic agent to molecular target drugs, antibody-drug conjugate (ADC), and immune-checkpoint inhibitors. Our laboratory established next generation PK/PD/PGx analyses to achieve Precision Medicine of antibody drugs. Our novel analyses include precise PK/PD analyses of antibody drugs, immune monitoring system, molecular drug imaging system, and originally developed NGS PGx panel. Moreover, by collaborating clinician and clinical pharmacologist, we are working on translational research of drug discovery & development.
Molecular Drug Imaging project
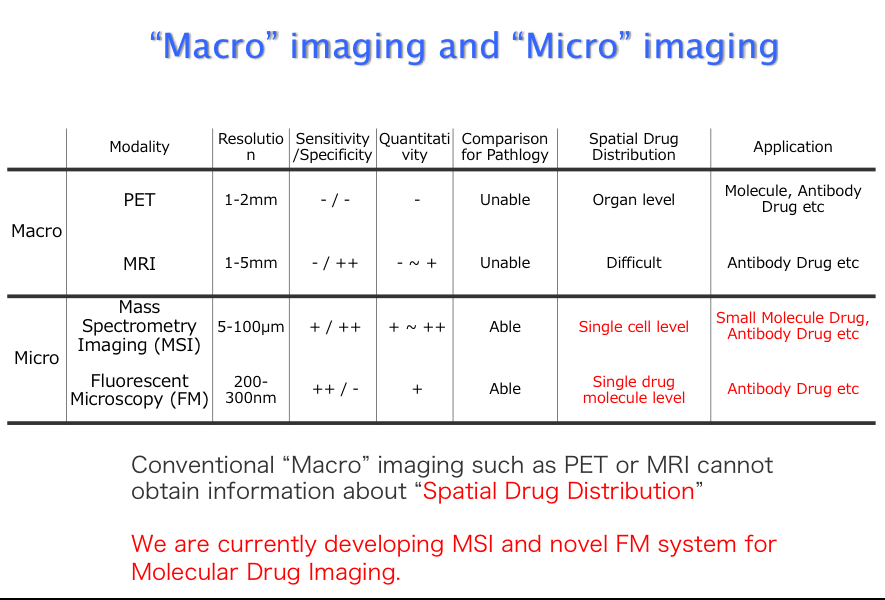
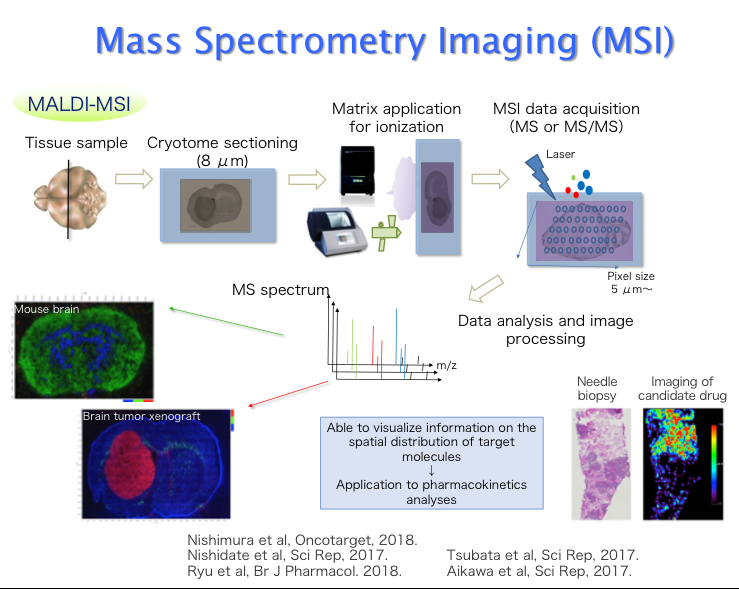
Molecular imaging is the medical practice and laboratory science of visualizing a molecular process in living body. Our laboratory focused on molecular process of anticancer drugs and corresponding various factors. We already developed Liquid Chromatography / Mass Spectrometry (LC-MS/MS) imaging system (MSI). By using MSI, we published many reports about spatial drug distribution, intra-tumoral drug concentration, and intra-tumoral drug heterogeneity. Moreover, we are also struggling with drug concentration analysis at a cell level. In cooperation with KONIKA-MINOLTA, we employed newly developed fluorescent nano-particle (PID). Using PID method, we expect to clarify the precise mode of action in antibody drugs.
J-PDX library project

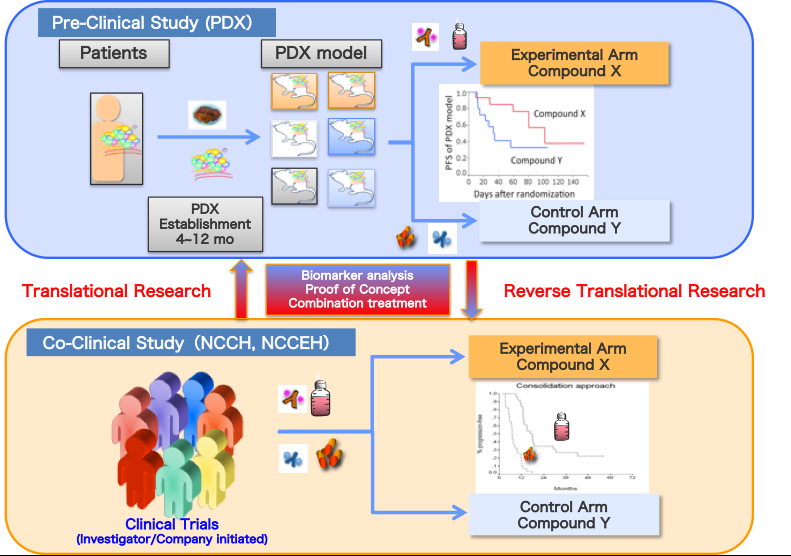
In the course of drug development, pre-clinical screening model is the key to success in development. In the past decades, cancer derived cell lines have been used as de facto standard for anticancer drug screening. NCI-60 cell lines derived from National Cancer Institute (NCI) are the most famous and frequently used screening model. However, since the cell line model showed low predictability of clinical efficacy (~5%), NIH decided to replace pre-clinical screening model from NCI-60 to patient-derived xenograft (PDX) model. PDX model is a mouse model which is established by simple transplantation of cancer tissue to an immunodeficient mouse. In recent years, PDX model has been thought as a better pre-clinical screening model since PDX possess and maintain tumor heterogeneity, tumor microenvironment, and original characteristics of donor tumor.
NCI already established more than 1,000 PDX models and started to distribute PDX models. EurOPDX consortium is also collecting various kinds of cancer PDXs in EU. In Japan, there is a limited number of PDXs such as Fukushima-project and Kanazawa university. For the purpose of drug development from Japan, construction of Japanese PDX library is urgently needed.
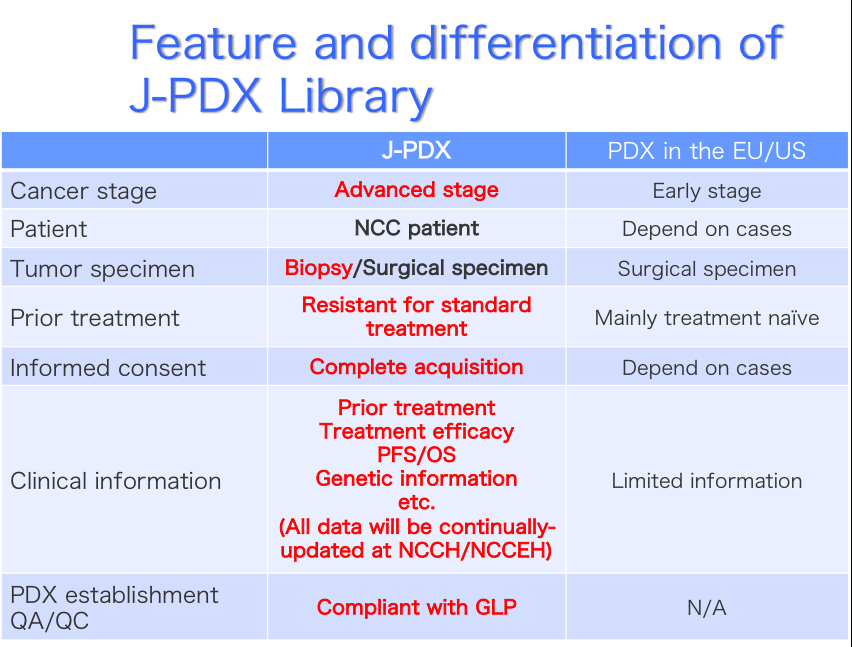
We National Cancer Center (NCC), National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN), and LSI Medience Corporation (LSIM) started Japanese Cancer Patient-derived Xenograft (J-PDX) Repository Establishment Program supported by the Japan Agency for Medical Research and Development (AMED) on March 7, 2018.
The objective of this program is to create an international-level research repository of Japanese Patient-Derived Xenografts (J-PDX) for use in the pharmaceutical industry in addition to nurturing expert personnel to promote academia-industry collaboration in their relevant fields. Three parties collaborate on this project: 1) the NCC which has two National Core Clinical Hospitals and Japan’s largest oncology research institute, 2) the NIBIOHN which was the first to establish a variety of PDX models in severe-combined immunodeficient mice and possesses an international-level biomedical institute, and 3) LSIM which has GLP facilities accredited by the Minister of Health, Labor and Welfare and serves as the sole Japanese CRO with the PDX expertise.
Following the requests of the Pharmaceuticals and Medical Devices Agency (PMDA), we are planning to 1) refine bio-ethical rules for the industrial use of J-PDXs, 2) establish a world-class J-PDX repository that includes major cancers (lung, colon, breast, stomach, uterus) in addition to prostate, pancreatic and rare cancers, along with the associated donors’ clinical information, 3) set criteria for the establishment and use of the J-PDX repository, 4) create standard operating procedures for the storage and use of J-PDXs in GLP-compliant, non-clinical studies, and 5) establish a research platform for fundamental and applied PDX science.

