Home > Organization > Division of Experimental Therapeutics (Tsukiji)
Group. of Clinical ResearchDivision of Experimental Therapeutics (Tsukiji)
News
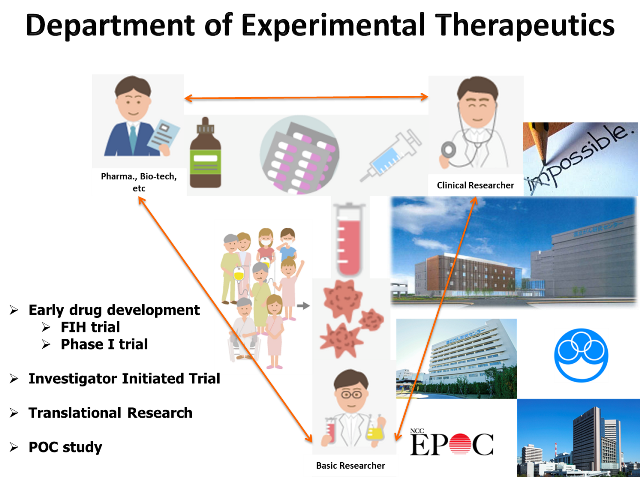
The department of Experimental Therapeutics supports efforts toward the creation of new drugs and other products from breakthroughs originating from academic institutions by achieving results at the level of basic research. We evaluate and discover excellent research and development proposals, and provide integrated management of the program so that basic research outcomes are linked to clinical research and clinical trial for approval. We have conducted several investigator initiated trials (IITs) as applications for approval and also have promoted research and development in the field of medicine. The top priority is to conduct first in human (FIH) trials, and we also perform phase I trials for solid tumors (i.e., all comers). Recently, we have been joining global phase I trials to accelerate the new drug development in Japan. Also, we promote the following activities for appropriate conducting of clinical research and trials.
- To plan the clinical trial as IIT for achieving proof of concept (POC) of new academic seeds
- Explanation meetings for researchers and office employees on compliance of laws, ordinances and guidelines
- To consider development design to create novelty with the goal of creating innovative drugs, medical devices, as well as ensuring that research projects on promising results proceed more rapidly and in greater depth