Home > Organization > Division of Translational Genomics (Tsukiji)
Group of Translational ResDivision of Translational Genomics (Tsukiji)
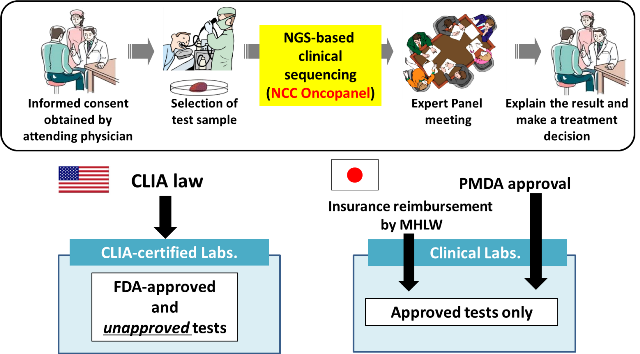
Next-generation sequencing (NGS) of tumor sample DNA (i.e., clinical sequencing) can guide clinical management by providing diagnostic or prognostic data, and facilitating the identification of potential treatment regimens, such as molecular-targeted and immune checkpoint blockade therapies (Kohno, Cancer Sci, 2018). However, tumor-profiling gene panel tests have not yet been implemented in routine oncological practice. Due to the lack of CLIA (Clinical Laboratory Improvement Amendments)-like regulation, such tests must be approved by the Pharmaceuticals and Medical Devices Agency (PMDA) before insurance reimbursement can be approved by the Japanese Ministry of Health, Labor, and Welfare (MHLW). By conducting a prospective cohort study (TOP-GEAR project; UMIN000011141), we have been implementing NGS-based gene-panel tests in routine oncological practice in Japan. We developed a NGS-based gene-panel test (NCC Oncopanel system) to examine somatic and germline mutations of 114 cancer-associated genes in advanced solid tumors. By using this system, we have shown that about 10% of examined cases have since received molecular-targeted therapy according to their gene aberrations (Sunami et al, Cancer Sci, 2019).
The NCC Oncopanel system was approved by PMDA as the first tumor-profiling gene panel tests in Japan on December 25, 2018, and its use is reimbursed by the National Health Insurance system.
At present, a gap exists between the number of patients with actionable mutations and those receiving genomically matched therapy. This gap is largely attributable to the lack of availability/accessibility of relevant trials and drugs. To fill the gap, we are collaborating with oncologists for the three purposes; 1) To facilitate molecularly driven clinical trials by developing tumor genome profiling tests, 2) To annotate VUSs (variants of unknown significance mutations) in druggable genes, and 3) To establish clinical trials for gene alterations that had been “undruggable”, such as deleterious mutations in the chromatin regulator genes.
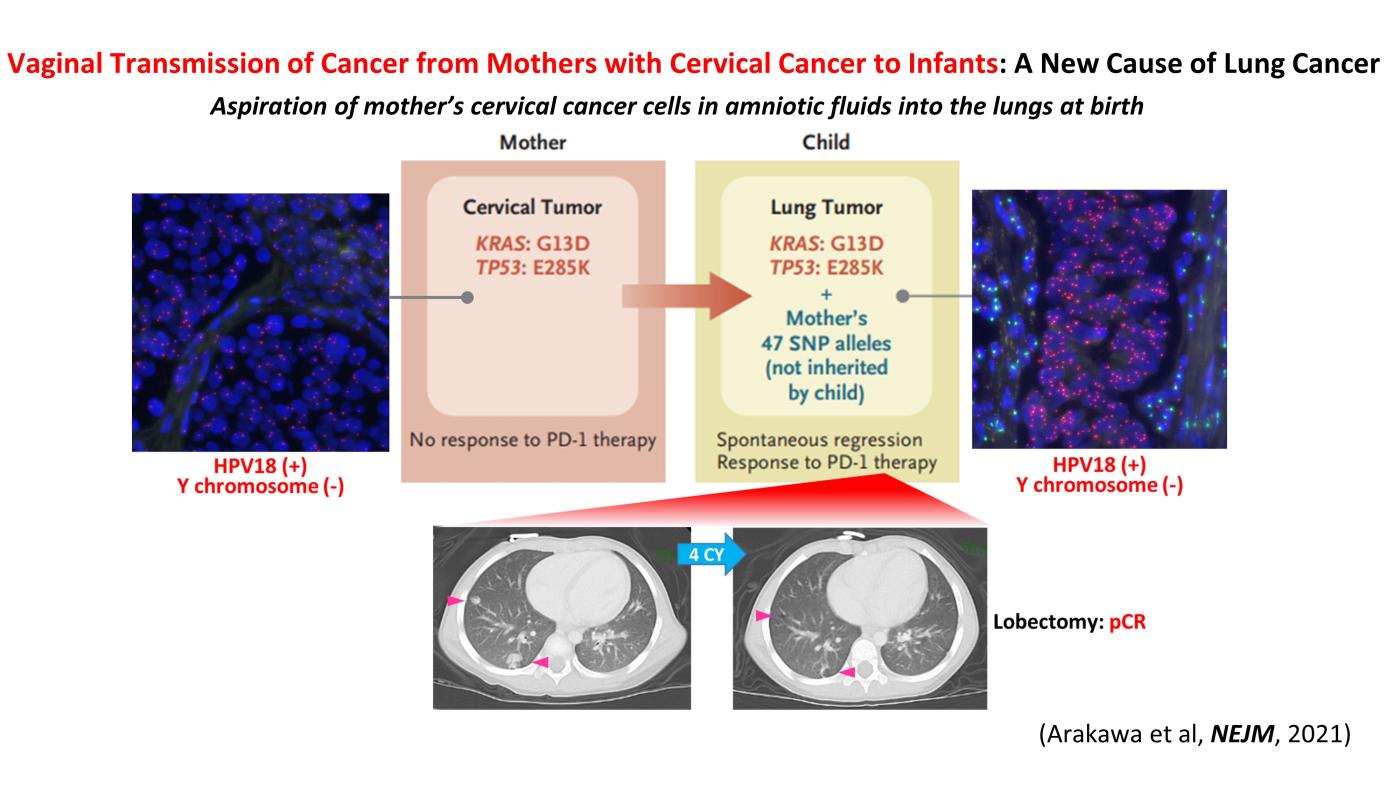
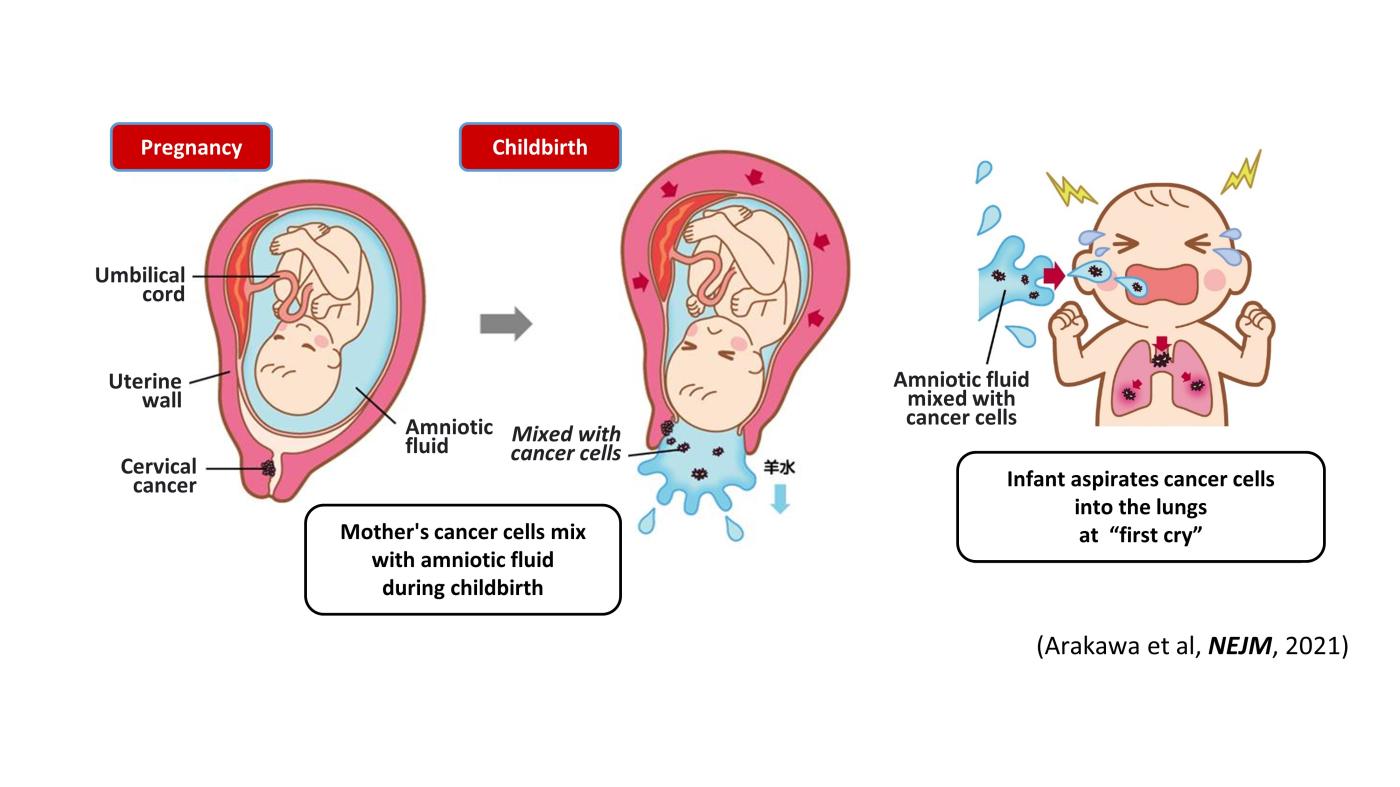
Two cases of pediatric lung cancer (in 23-month-old and 6-year-old boys) resulting from mother-to-infant transmission of uterine cervical tumors were incidentally detected during routine NCC Oncopanel testing of paired samples of tumor and normal tissue. Spontaneous regression of some lesions in the first child and slow growth of the tumor mass in the second child suggested the existence of alloimmune responses against the transmitted tumors. Immune checkpoint inhibitor therapy with nivolumab led to a strong regression of all remaining tumors in the first child (Arakawa et al, NEJM, 2021).