Home > Information > press release > CIRCULATE-Japan GALAXY Confirms Effectiveness of Liquid Biopsy in Predicting Recurrence Risk and Post-operative Treatment Efficacy in Colorectal Cancer
CIRCULATE-Japan GALAXY Confirms Effectiveness of Liquid Biopsy in Predicting Recurrence Risk and Post-operative Treatment Efficacy in Colorectal Cancer-Published in Nature Medicine-
Key Points
- The CIRCULATE-Japan*1 GALAXY study*2 involving 152 institutions in Japan and abroad examined liquid biopsy*3 results and recurrence risks for 2,240 colorectal cancer patients who underwent surgery.
- Patients whose post-operative blood tests showed evidence of cancer (circulating tumor DNA*4 [ctDNA]) had a higher risk of cancer recurrence and shorter survival periods compared to those without detectable ctDNA.
- Recurrence risk could be predicted from ctDNA, independently from cancer genetic information (biomarkers*5).
- For patients with detectable ctDNA after surgery, those who received post-operative chemotherapy (adjuvant chemotherapy)*6 and subsequently cleared ctDNA had a lower risk of recurrence.
- These findings suggest that ctDNA testing can predict post-surgical outcomes for colorectal cancer patients more precisely, enabling personalized post-surgical treatment. This is expected to improve treatment outcomes for a greater number of colorectal cancer patients.
Overview
A research group led by Dr. Takayuki Yoshino, Deputy Director, and Dr. Yoshiaki Nakamura, Chief of the International Research Promotion Office at National Cancer Center Hospital East (Director: Toshihiko Doi, Kashiwa City, Chiba Prefecture) of the National Cancer Center (President: Hitoshi Nakagama, Chuo-ku, Tokyo), along with Associate Professor Eiji Oki from Kyushu University (President: Tatsuro Ishibashi, Fukuoka City, Fukuoka Prefecture), investigated the relationship between liquid biopsy results and cancer recurrence risk and survival time for 2,240 patients with colorectal cancer participating in the CIRCULATE-Japan GALAXY trial.
The results showed that patients with detectable circulating tumor DNA (ctDNA) in post-operative blood tests had a higher risk of cancer recurrence and shorter survival time compared to those without detectable ctDNA. Furthermore, it was revealed that even among patients with detectable ctDNA, those who received post-operative adjuvant chemotherapy and subsequently cleared ctDNA had a lower risk of recurrence.
These research findings scientifically demonstrate that examining ctDNA can predict the risk of colorectal cancer recurrence and survival time, aiding in treatment decision-making. This research was published in Nature Medicine on September 16, 2024 at 10 a.m. (London time).
Background
In the world of cancer treatment, liquid biopsies, which examine circulating tumor DNA (ctDNA) through blood tests, have gained attention. Liquid biopsies for genetic testing in patients with advanced cancer are already in clinical use in Japan. It was also known that examining ctDNA in patients who had undergone surgery could indicate the risk of cancer recurrence. However, there was insufficient evidence regarding the relationship between ctDNA and patient survival time, as well as its relationship with the effectiveness of post-operative adjuvant chemotherapy.
To address this, the National Cancer Center Hospital East initiated a large research project called CIRCULATE-Japan in 2020. This project aims to deliver appropriate treatment to patients with colorectal cancer undergoing surgery using ctDNA testing. The GALAXY trial, a part of CIRCULATE-Japan, examines blood samples from patients with colorectal cancer undergoing surgery to check for the presence of ctDNA.
In this study, using data from patients participating in the GALAXY trial, researchers thoroughly investigated how ctDNA test results relate to cancer recurrence risk and survival time. They also examined how changes in ctDNA levels are associated with the effectiveness of post-operative adjuvant chemotherapy.
Study Methods and Results
The study investigated the relationship between ctDNA test results, cancer recurrence risk, and survival time for 2,240 patients with colorectal cancer participating in the CIRCULATE-Japan GALAXY trial.
In the GALAXY trial, blood samples collected from patients were examined for the presence of ctDNA. The ctDNA testing used Signatera, a cutting-edge technology developed by Natera Inc. in the United States. To closely monitor changes in ctDNA, blood samples were collected before surgery and regularly starting from 4 weeks after surgery.
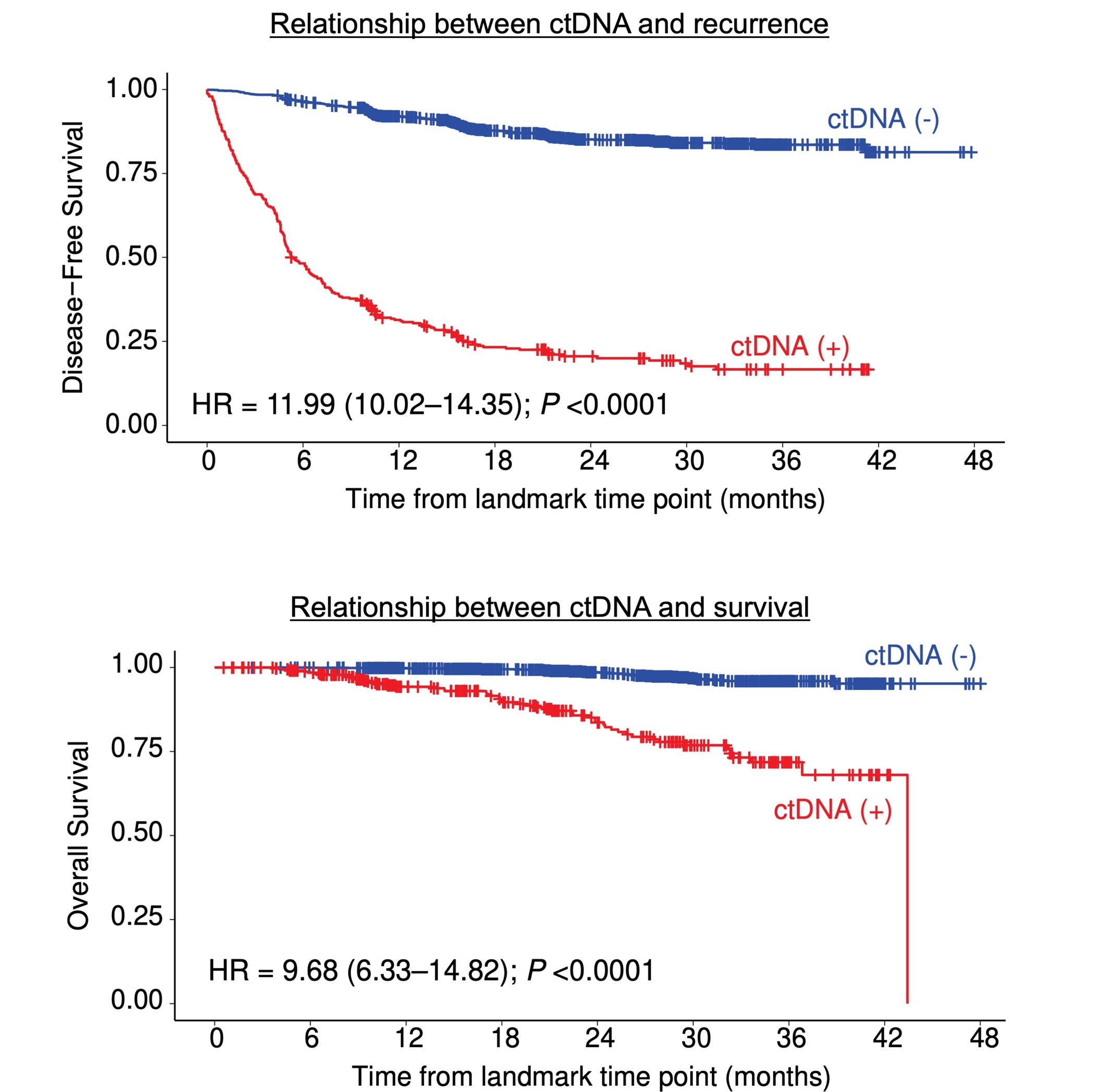
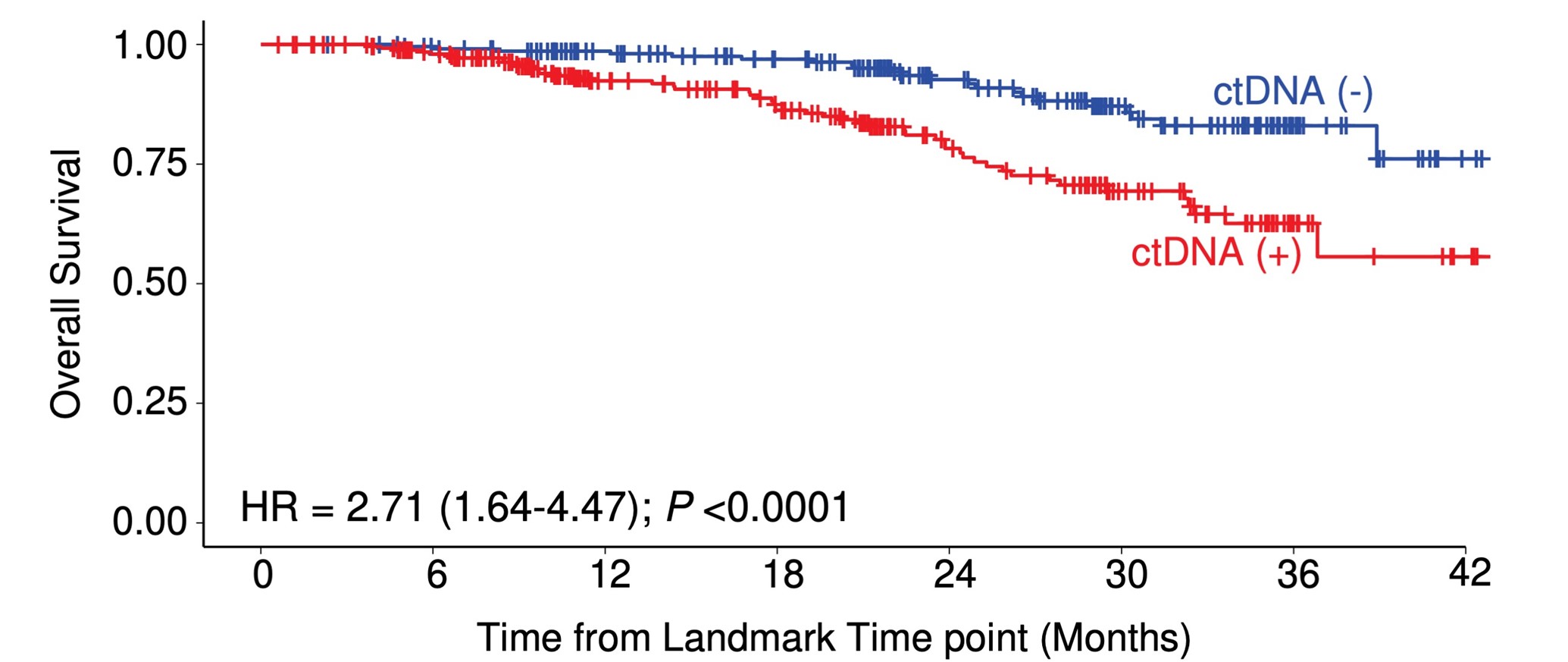
The research results showed that patients who tested positive for ctDNA 2-10 weeks after surgery had approximately 12 times higher risk of cancer recurrence compared to those who tested negative. Moreover, there was a significant difference in the proportion of patients without cancer recurrence after two years: 20.6% for ctDNA-positive patients versus 85.1% for ctDNA-negative patients. Overall survival rates also differed, with 83.7% for ctDNA-positive patients and 98.5% for ctDNA-negative patients after two years (Figure 1). Interestingly, even among patients who experienced cancer recurrence, there was a difference in survival time between ctDNA-positive and ctDNA-negative patients (Figure 2). These results demonstrate that post-operative ctDNA testing is highly valuable in predicting recurrence risk and survival time for patients with colorectal cancer.
Figure 1: Relationship between post-operative ctDNA and cancer recurrence/survival time
Figure 2: Relationship between post-operative ctDNA and survival time in recurrent patients
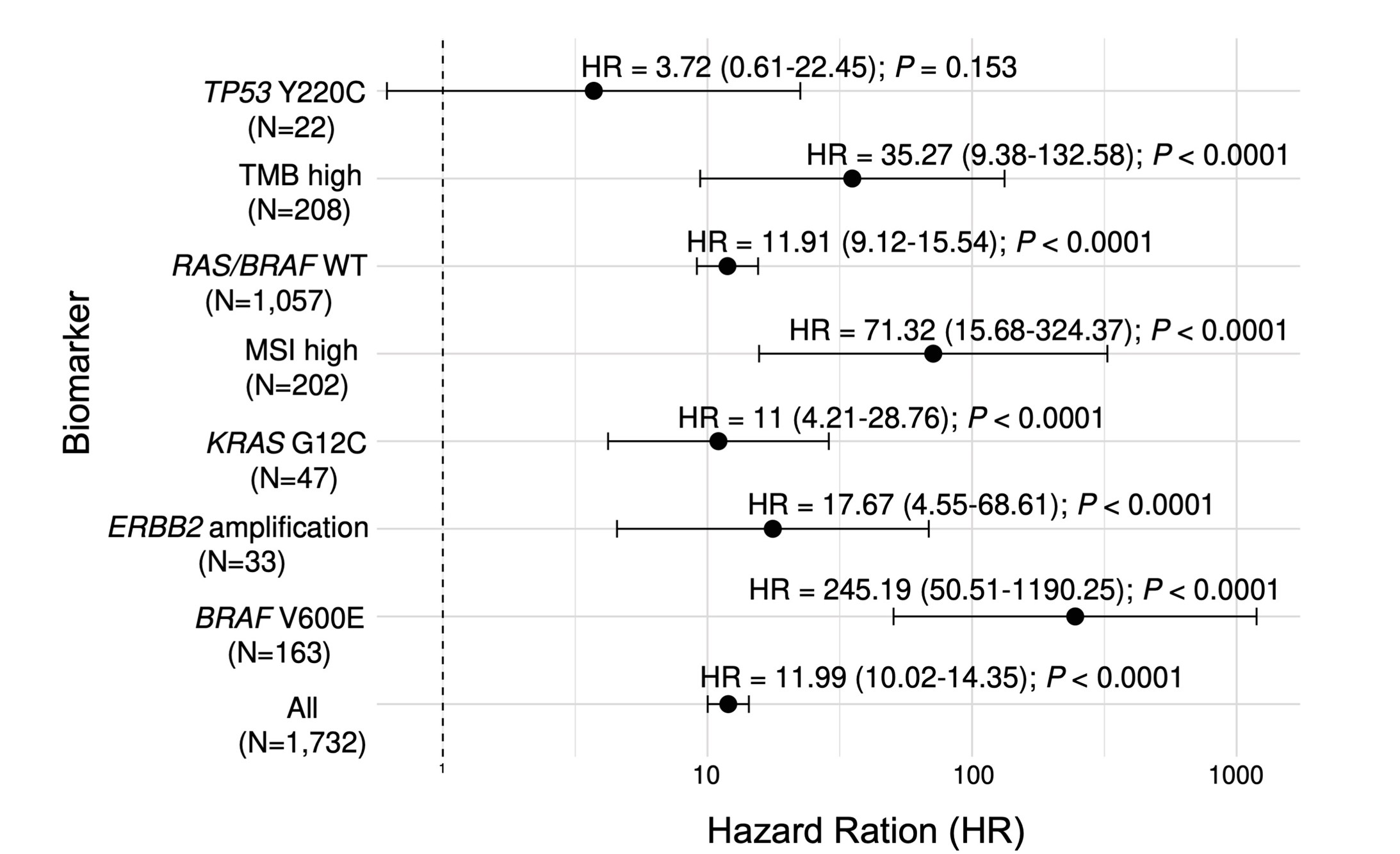
Recent studies have shown that colorectal cancers have different characteristics based on their genomic information (biomarkers). Therefore, we examined the relationship between ctDNA test results and recurrence risk for each of the major biomarkers in colorectal cancer. The biomarkers studied included TP53 Y220C mutation, high tumor mutational burden (TMB high), RAS/BRAF wild type (RAS/BRAF WT), high microsatellite instability (MSI high), KRAS G12C mutation, ERBB2 gene amplification (ERBB2 amplification), and BRAF V600E mutation. The research revealed that regardless of the biomarker, patients who were ctDNA-positive had a higher risk of recurrence compared to those who were ctDNA-negative (Figure 3). This finding suggests that ctDNA testing can be a powerful tool for predicting recurrence risk, regardless of the cancer's genetic characteristics. These results indicate that ctDNA testing may be useful in determining treatment strategies based on individual patients' genetic characteristics.
Figure 3. Post-operative ctDNA and cancer recurrence for each biomarker
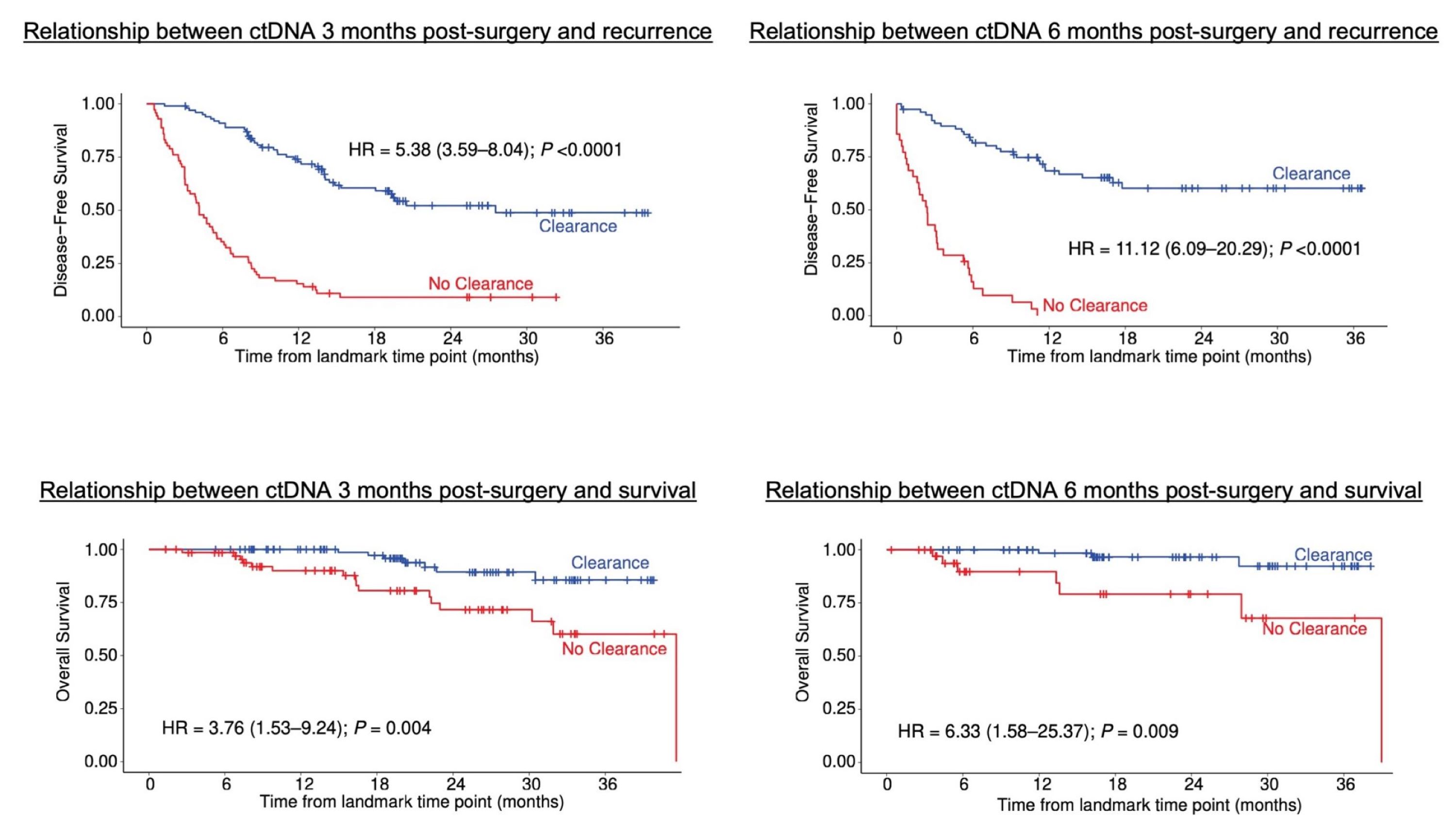
Lastly, we examined changes in ctDNA in ctDNA-positive patients who received post-operative adjuvant chemotherapy. Previous reports from the GALAXY trial had shown that adjuvant chemotherapy was effective in ctDNA-positive patients, while its effect was unclear in ctDNA-negative patients. In this study, we conducted a more detailed analysis to investigate the changes in ctDNA due to adjuvant chemotherapy and its impact. The results showed that among ctDNA-positive patients who received adjuvant chemotherapy, some patients' ctDNA became undetectable (cleared) at 3 or 6 months post-surgery (Figure 4). Importantly, it was revealed that patients whose ctDNA cleared tended to have a lower risk of recurrence and longer survival time. This finding suggests the possibility of evaluating the effectiveness of adjuvant chemotherapy at an early stage. In other words, regular ctDNA testing may allow for determining whether the treatment is working effectively and adjusting the treatment plan if necessary.
Figure 4: Relationship between ctDNA clearance at 3 and 6 months post-surgery and cancer recurrence/survival time
Perspectives
The results of this study could represent a significant step towards personalized treatment for colorectal cancer.
Firstly, the potential for more accurate prediction of colorectal cancer recurrence risk using ctDNA testing may allow for more appropriate treatment plans tailored to each patient.
Additionally, the possibility of early assessment of treatment effectiveness through regular ctDNA testing could be useful in evaluating the efficacy of new treatments at an early stage. If treatment effectiveness can be determined early, it may be possible to discontinue ineffective treatments sooner or switch to alternative treatments.
In the future, if such tests are approved, they are expected to be widely used in clinical settings. This would allow many patients with colorectal cancer to benefit from this technology.
Furthermore, CIRCULATE-Japan is conducting important clinical trials (ALTAIR trial and VEGA trial) to verify these research results. The ALTAIR trial (JapicCTI-2053) is a randomized controlled phase 3 study for ctDNA-positive patients, while the VEGA trial (jRCT1031200006) is a randomized controlled phase 3 study for patients who are ctDNA-negative at 4 weeks post-surgery. The results of these trials are expected to further confirm the clinical usefulness of ctDNA testing and lead to future approval and widespread use.
To extend the insights gained from CIRCULATE-Japan to cancer patients beyond colorectal cancer, a new large-scale study "SCRUM-Japan MONSTAR-SCREEN-3" has been launched. This study will expand the target group to patients with solid tumors and hematological malignancies (blood cancers), and will conduct cutting-edge multi-omics analysis*7 including liquid biopsy. We will continue to utilize world-leading analysis techniques and work tirelessly to advance personalized cancer medicine, to deliver effective treatments to cancer patients and their families worldwide.
Publication
Journal
Nature Medicine
Title
ctDNA-based molecular residual disease and survival in resectable colorectal cancer
Authors
Yoshiaki Nakamura†, Jun Watanabe†, Naoya Akazawa, Keiji Hirata, Kozo Kataoka, Mitsuru Yokota, Kentaro Kato, Masahito Kotaka, Yoshinori Kagawa, Kun-Huei Yeh, Saori Mishima, Hiroki Yukami, Koji Ando, Masaaki Miyo, Toshihiro Misumi, Kentaro Yamazaki, Hiromichi Ebi, Kenji Okita, Atsushi Hamabe, Hiroki Sokuoka, Satoshi Kobayashi, George Laliotis, Vasily N. Aushev, Shruti Sharma, Adham Jurdi, Minetta C. Liu, Alexey Aleshin, Matthew Rabinowitz, Hideaki Bando, Hiroya Taniguchi, Ichiro Takemasa, Takeshi Kato, Daisuke Kotani, Masaki Mori, Takayuki Yoshino*, Eiji Oki*
(† Contribution equal to first author, * corresponding author)
DOI
10.1038/s41591-024-03254-6
Date
September 16, 2024 at 10 a.m. (London time)
URL
https://www.nature.com/articles/s41591-024-03254-6 (linked at external site)
Research Funding
Japan Agency for Medical Research and Development (AMED)
Research Program: Practical Research for Innovative Cancer Control
Research Project: A Basket-Type Clinical Trial of TAS-120 for Refractory Advanced Solid Tumors with FGFR Alterations in Circulating Tumor DNA utilizing SCRUM-Japan Cancer Genome Screening Platform
Platform Principal Investigator: Takayuki Yoshino
Glossary
*1 CIRCULATE-Japan
 A project aiming at accurately estimating the post-operative recurrence risk for patients undergoing surgical treatment for colorectal cancer using the latest liquid biopsy analysis technology, to provide more appropriate medical care. About 150 facilities (including one overseas) participate. It consists of large-scale investigator-initiated international clinical trials (GALAXY, VEGA, ALTAIR), with the GALAXY trial serving as the core study.
A project aiming at accurately estimating the post-operative recurrence risk for patients undergoing surgical treatment for colorectal cancer using the latest liquid biopsy analysis technology, to provide more appropriate medical care. About 150 facilities (including one overseas) participate. It consists of large-scale investigator-initiated international clinical trials (GALAXY, VEGA, ALTAIR), with the GALAXY trial serving as the core study.
◆Reference Press Releases:
・June 10, 2020 "Start of New Project "CIRCULATE-Japan" Aiming to Realize Personalized Cancer Medicine with Liquid Biopsy-World's Largest Physician-Initiated International Clinical Trial for Invisible Cancer-"
https://www.ncc.go.jp/jp/information/pr_release/2020/0610/index.html
*2 GALAXY study
A core study among the three investigator-initiated international clinical trials in the CIRCULATE-Japan project. It analyzes ctDNA longitudinally before and after colorectal cancer surgery to investigate its association with recurrence and survival periods.
◆Reference Press Releases:
・January 24, 2023 "Clinical Utility of Circulating Tumor DNA After Surgery for Colorectal Cancer Demonstrated in a Large-Scale Prospective Study -A Step toward Personalized Postoperative Adjuvant Chemotherapy-”
https://www.ncc.go.jp/jp/information/pr_release/2023/0124/index.html
*3 Liquid Biopsy
A test that detects cancer genomic abnormalities using a patient's blood. As it can be repeatedly measured through blood tests, it is less burdensome on the body and is expected to detect cancer recurrence at an earlier stage.
*4 Circulating tumor DNA
Cancer-derived DNA present in very small amounts in the blood.
*5 Biomarker
An indicator that can objectively measure and evaluate biological changes in the body. In cancer care, genetic information of cancer and protein levels are used to diagnose cancer and predict treatment effectiveness.
*6 Post-operative adjuvant chemotherapy
Anticancer drug treatment given after cancer surgery. It is performed to eliminate microscopic cancer cells that may remain after surgery and to reduce the possibility of recurrence.
*7 Multi-omics analysis
An analytical method that comprehensively analyzes genomics, transcriptomics, proteomics, metabolomics, etc. The suffix "-omics" signifies comprehensive analysis.
Inquiries
On Research
National Cancer Center Hospital East
SCRUM-Japan Office
E-mail: scrum_office●east.ncc.go.jp
From Media
National Cancer Center (Kashiwa Campus)
Office of Public Relations, Strategic Planning Bureau,
E-mail: ncc-admin●ncc.go.jp
Kyushu University
Public Relations Initiative
Phone: 092-802-2443
Email: sysintlkh●jimu.kyushu-u.ac.jp