Home > Information > press release > SCRUM-Japan GOZILA Confirms Extended Survival with Personalized Cancer Treatment in 4,000+ Patients Study
SCRUM-Japan GOZILA Confirms Extended Survival with Personalized Cancer Treatment in 4,000+ Patients Study
September 17, 2024
National Cancer Center
Key points
- SCRUM-Japan GOZILA ProjectNote1 examined liquid biopsy results /treatment outcomes for 4,037 patients with advanced cancer.
- 24% patients received targeted therapy based on liquid biopsy results.
- Patients who received liquid biopsy-guided treatment survived approximately twice as long as those who did not.
- This study demonstrates that personalized cancer treatment using liquid biopsy can significantly extend patient survival.
- These findings are expected to advance liquid biopsy-guided cancer treatment and improve outcomes for more patients.
Overview
A research group led by Takayuki Yoshino, Deputy Director of the National Cancer Center (President: Hitoshi Nakagama, Chuo-ku, Tokyo) Hospital East (Hospital Director: Toshihiko Doi, Kashiwa City, Chiba Prefecture), investigated the effects of personalized treatment based on liquid biopsies in 4,037 patients with advanced cancer who participated in the SCRUM-Japan GOZILA project.
The study found that 24% of participants received personalized targeted therapy based on their liquid biopsy results. Importantly, patients who received targeted treatment guided by liquid biopsies lived approximately twice as long as those who did not.
These results scientifically demonstrate that personalized cancer treatment using liquid biopsies can significantly extend patient survival. This research was published in Nature Medicine on September 16, 2024 at 10 a.m. (London time).
Background
In recent years, personalized cancer treatments based on individual patients' genomic information have gained attention as a means of improving cancer care.
Liquid biopsies, which examine tumor DNA in blood samples, is expected to be a key method for realizing personalized cancer treatments.
Compared to conventional tissue biopsies, liquid biopsies have several advantages: they are less invasive to patients, allowing for repeated testing, and can simultaneously examine cancer characteristics from different parts of the body. However, until now, it was unclear whether treatments using liquid biopsies actually benefited patients. In particular, there was insufficient evidence on how long patients survived when choosing treatments based on this method.
The SCRUM-Japan GOZILA Project, initiated in 2018 as part of the SCRUM-Japan MONSTAR-SCREEN industry-academia cancer genomic screening project led by the National Cancer Center Hospital East, has been working to deliver appropriate treatments to as many patients as possible using liquid biopsies, with the cooperation of many patients.
In this study, we examined the effects of treatments conducted in the SCRUM-Japan GOZILA Project. Through this investigation, we confirmed whether treatments selected using liquid biopsies (genomic analysis of fluid samples such as plasma and urine, which are less invasive to the body) are actually helping patients.
Research Methods and Results
This study investigated the effects of personalized treatment based on liquid biopsies in 4,037 advanced cancer patients who participated in the SCRUM-Japan GOZILA Project.
First, we performed liquid biopsies on blood samples collected from patients to examine their cancer genomic information (biomarkers). We used the Guardant360® CDx Cancer Genome Profiling test to analyze 74 cancer-related genes. Next based on the liquid biopsy results, appropriate targeted therapies were selected for patients. This provided treatment options that were not available through traditional methods. We then followed the progress of treated patients and analyzed their treatment response and survival time in detail.
The results showed that 24% of participants were able to receive targeted treatment tailored to them based on their liquid biopsy results (Figure 1). This demonstrates that liquid biopsy can provide new treatment opportunities for many patients.
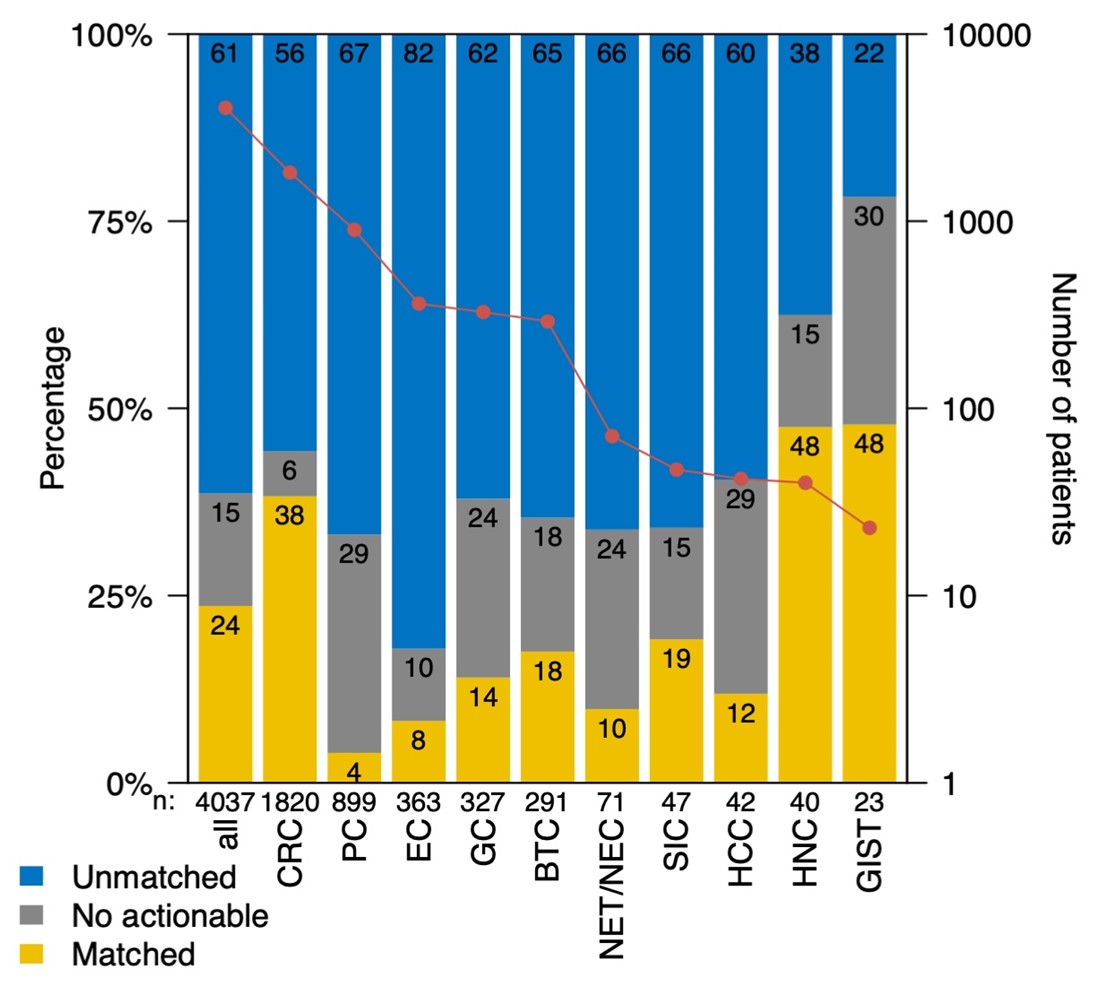
Figure 1: Percentage of patients treated with liquid biopsy-based therapy
An even more important finding was that patients who received liquid biopsy-based targeted therapy lived almost twice as long as those who did not. Specifically, patients who received targeted therapy had a median survival of 18.6 months compared to 9.9 months for those who did not (Hazard ratio 0.54, Figure 2). This indicates that personalized therapy guided by liquid biopsy has the potential to significantly extend patient survival. We also found that patients without treatment-linked actionable biomarkers (median survival: 16.8 months) had longer survival than patients with biomarkers who did not receive matching treatment (Hazard ratio 0.60, Figure 2). Given that genomic alterations are sometimes associated with treatment resistance, this suggests that patients without biomarkers may have longer survival because they were free of genomic alterations associated with treatment resistance. It is also known that some patients do not have tumor DNA in their blood and therefore no biomarkers detected by liquid biopsy, but such cases also tend to have longer survival.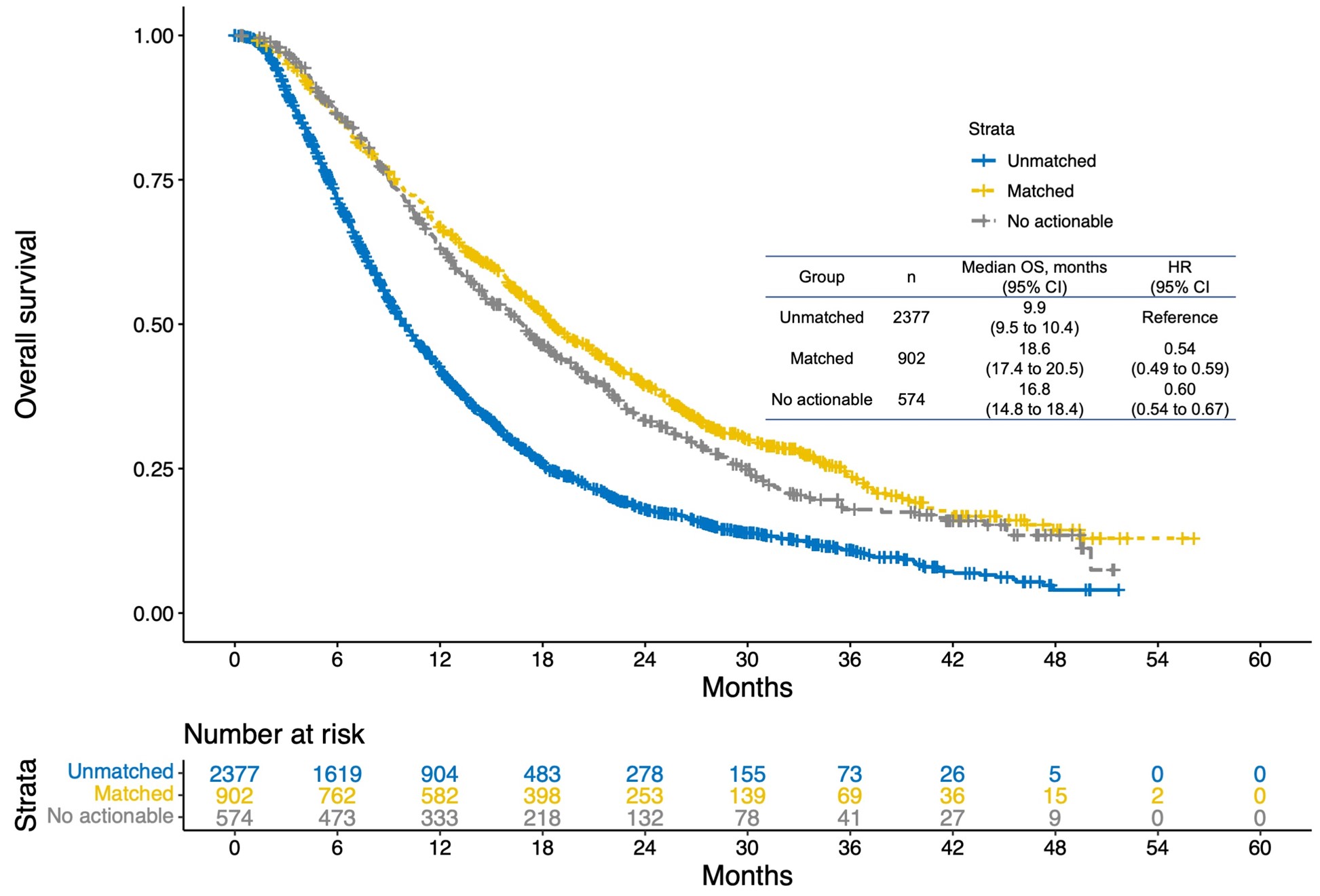
Figure 2: Targeted treatment based on liquid biopsy and overall survival
The study also analyzed the characteristics of biomarkers found in liquid biopsies. Results showed that treatment was more effective in cases with higher clonality (the proportion of cells with the mutation in total cancer cells) and higher adjusted plasma copy number (the plasma copy number of genes adjusted for the amount of cancer-derived DNA in the blood) (Figure 3). This finding may lead to more precise treatment selection in the future.
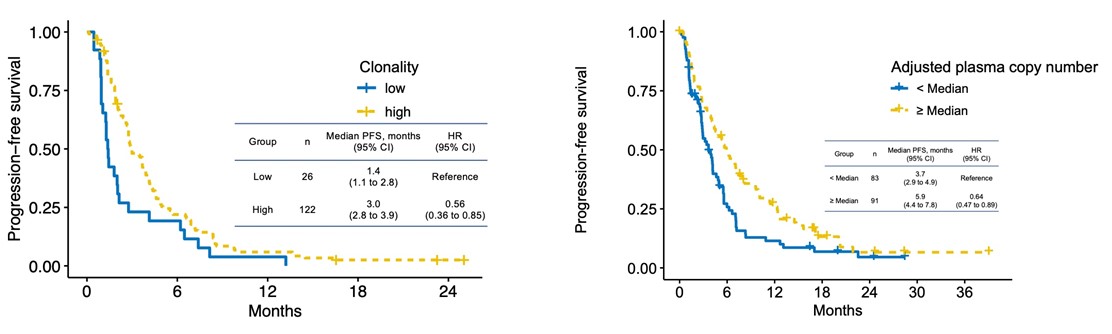
Figure 3. Clonality, adjusted plasma copy number, and progression-free survival of therapeutic target biomarkers
These results provide scientific evidence that personalized cancer treatment using liquid biopsies can extend patient survival. This study is the first to demonstrate the survival-extending effect of liquid biopsy-based personalized cancer treatment on a large scale across various cancers and is expected to contribute significantly to future advances in cancer treatment.
Future perspective
The results of this study have the potential to bring about a paradigm shift in cancer treatment. The scientific demonstration of the efficacy of personalized cancer treatment using liquid biopsies suggests a future direction for cancer medicine.
First, the clinical application of liquid biopsy is expected to expand further. If the usefulness of this technology is established, it will increase the possibility of providing optimized personalized treatment to more patients.
Further research on the relationship between biomarker characteristics (clonality and adjusted plasma copy number) suggested in this study and treatment efficacy may enable more accurate prediction of treatment outcomes.
On the other hand, there are several challenges to address. For example, reducing the cost of liquid biopsy testing, educating healthcare professionals on interpretation of test results, and expanding insurance coverage, they are all important issues.
The SCRUM-Japan MONSTAR-SCREEN project is planning a new large-scale study, "MONSTAR-SCREEN-3." This study plans to expand its scope beyond patients with advanced solid tumors to include patients with early-stage solid tumors and hematological malignancies who can undergo curative resection, and to perform cutting-edge multi-omics analysisNote2 including liquid biopsy. We will continue to devote ourselves to the development of personalized cancer medicine, utilizing the world's most advanced analysis techniques to deliver effective therapies to cancer patients and their families worldwide.
Journal Information
Journal Name
Nature Medicine
Title
Targeted therapy guided by circulating tumor DNA analysis in advanced gastrointestinal tumors: SCRUM-Japan GOZILA
Authors
Yoshiaki Nakamura, Hiroshi Ozaki, Makoto Ueno, Yoshito Komatsu, Satoshi Yuki, Taito Esaki, Hiroya Taniguchi, Yu Sunakawa, Kensei Yamaguchi, Ken Kato, Tadamichi Denda, Tomohiro Nishina, Naoki Takahashi, Taroh Satoh, Hisateru Yasui, Hironaga Satake, Eiji Oki, Takeshi Kato, Takashi Ohta, Nobuhisa Matsuhashi, Masahiro Goto, Naohiro Okano, Koushiro Ohtsubo, Kentaro Yamazaki, Riu Yamashita, Naoko Iida, Mihoko Yuasa, Hideaki Bando, Takayuki Yoshino*
*Corresponding author
DOI
10.1038/s41591-024-03244-8
Publication date
September 16, 2024 at 10 a.m. (London time)
URL
https://www.nature.com/articles/s41591-024-03244-8
Research expenses
Grant-in-aid
National Cancer Center
Research project
National Cancer Center Research Fund
Title
Research on human resource development and infrastructure for clinical application of whole exome, genome, and multi-omics analysis
Research Representative
Takayuki Yoshino
Issue Number
2021 A-6
Please also refer to the following press releases for the latest SCRUM-Japan research.
- Press Release dated August 6 ”Cross-organ efficacy of trastuzumab deruxtecan confirmed against solid tumors with HER2 gene amplification confirmed by liquid biopsy- Results of the industry-academia collaboration SCRUM-Japan MONSTAR project published in Journal of Clinical Oncology, flagship journal of the American Society of Clinical Oncology –“ (in Japanese)
https://www.ncc.go.jp/jp/information/pr_release/2024/0806/index.html
(Source)
Trastuzumab Deruxtecan in Advanced Solid Tumors With Human Epidermal Growth Factor Receptor 2 Amplification Identified by Plasma Cell-Free DNA Testing: A Multicenter, Single-Arm, Phase II Basket Trial (Published August 01, 2024)
https://ascopubs.org/doi/10.1200/JCO.23.02626 (linked at external site) - Press Release dated July 19 "The World's Largest Integrated Analysis Confirms Prolonged Survival with Personalized Cancer Therapy ― Results of SCRUM-Japan MONSTAR Project Published in Cancer Discovery ―"
https://www.ncc.go.jp/en/information/press_release/20240719/index.html - Press Release dated July 19 "Anti-EGFR Antibody Drug Discovers Potential New Therapeutic Target - New Treatment Option for Colorectal Cancer Patients with RAS Mutation Changed to Wild-Type after Drug Therapy - " (in Japanese)
https://www.ncc.go.jp/jp/information/pr_release/2024/0719_2/index.html
(Source)
Clinical features associated with NeoRAS wild-type metastatic colorectal cancer A SCRUM-Japan GOZILA substudy (Published: 13 July 2024)
https://www.nature.com/articles/s41467-024-50026-4 (linked at external site)
Glossary
(Note1) SCRUM-Japan GOZILA Project
SCRUM-Japan is an industry-academia collaborative cancer genome screening project that integrates LC-SCRUM-Japan (currently LC-SCRUM-Asia), which investigates cancer genomic alterations in lung cancer patients, launched in 2013, and GI-SCREEN-Japan (currently MONSTAR-SCREEN), which conducts cancer genomic analyses in gastrointestinal cancer patients, launched in 2014. The GOZILA project, launched in 2018 as part of the SCRUM-Japan MONSTAR-SCREEN project, is conducting comprehensive cancer genome profiling using liquid biopsy for gastrointestinal cancer and other cancer patients.
(Note2) Multi-omics analysis
This is a comprehensive analysis method for genomic analysis (genomics), RNA analysis (transcriptomics), protein analysis (proteomics), and metabolite analysis (metabolomics). "-omics" means comprehensive analysis.
https://phambielinq.com/ (linked at external site)
Inquiries
For Research
SCRUM-Japan Office
National Cancer Center Hospital East
Phone: 04-7133-1111 (Main line)
Email: scrum_office●east.ncc.go.jp
For media
Office of Public Relations, Strategic Planning Bureau
National Cancer Center
Phone: 04-7133-1111 (Main line)
E-mail: ncc-admin●ncc.go.jp
