Home > Information > press release > Launch of “ARCAD-Gastric”, a global gastric cancer clinical trial database project
Launch of “ARCAD-Gastric”, a global gastric cancer clinical trial database project-Expansion of the database from colorectal cancer to gastric cancer to promote further research and development of cancer treatment-
January 31, 2025
National Cancer Center Japan
The ARCAD Foundation
Mayo Clinic
Key Points of the announcement
- The National Cancer Center Hospital East (“Hospital East”), the ARCAD Foundation, and Mayo Clinic have launched a gastric cancer database project, ARCAD-Gastric, to collect, integrate and utilize clinical trial data on gastric cancer worldwide and the three parties have signed an agreement.
- The ARCAD Database Project, a project to collect, integrate, and utilize data from clinical trials and trials conducted in the past, has established a global database with 67 trials including approximately 47,000 colorectal cancer patients as of December 2024 and is utilizing the database. “ARCAD-Gastric” will be expanded from colorectal cancer to gastric cancer, and the database will be shared by three data centers in Japan, the U.S., and Europe, aiming to create a global joint research system and provide better medical care.
- In the future, by further expanding and sharing the database and establishing a system to utilize the data, we will lead the research and development of cancer treatment around the world.
Overview
The National Cancer Center (Japan), the ARCAD Foundation (France), and the Mayo Clinic (United States) have launched the “ARCAD-Gastric” project on October 30, 2024 and the three parties have signed an agreement. In the late 2000s, the ARCAD Foundation and the Mayo Clinic initiated the “ARCAD Database Project,” an integrated database project of clinical trial data on advanced recurrent colorectal cancer in the United States and Europe, and the database was expanded to Asia with the participation of Hospital East from 2021. ARCAD-Gastric has decided the following for the purpose of building a database of gastric cancer clinical trials, and will promote evidence generation and treatment development on a global scale by conducting various analyses of the constructed database.
・First, it covers pivotal phase II and III trials*1of immunotherapy, a treatment that uses the power of the immune system to attack cancer cells targeting approximately 10,000 cases in 11 trials on immune checkpoint inhibitors.
・Second, The data will be collected and evaluated for usefulness in terms of biomarkers such as PD-1/PD-L1 and MSI*2, which are necessary test items for diagnostic criteria of diseases and evaluation of therapeutic efficacy, as well as data on immune-related adverse events.
・Third, the constructed database will be used to make recommendations on surrogate endpoints in clinical trials and the regulatory approval process.
This project is managed by Prof. Thierry André (The ARCAD Foundation), Prof. Qian Shi (Mayo Clinic) and Prof. Takayuki Yoshino (Hospital East). Prof. Takayuki Yoshino, Deputy Director of the hospital, is the Principal Investigator of the project, and the ARCAD Asia Data Center*3 will lead the data integration of all global clinical trials. The structure of the database is planned to adopt the CDISC format, which is an international standard for clinical trial data on pharmaceuticals industry, so that it can be used in the future for applications for approval across cancer types and policy recommendation. The system and work procedures used for data construction are the same as those used to construct the colorectal cancer database. The constructed database will be transferred from the ARCAD Asia Data Centers to data centers in France and the United States, and is expected to be utilized by pharmaceutical companies and academia at each datacenter.
ARCAD-Gastric joint management system and Global DB flow 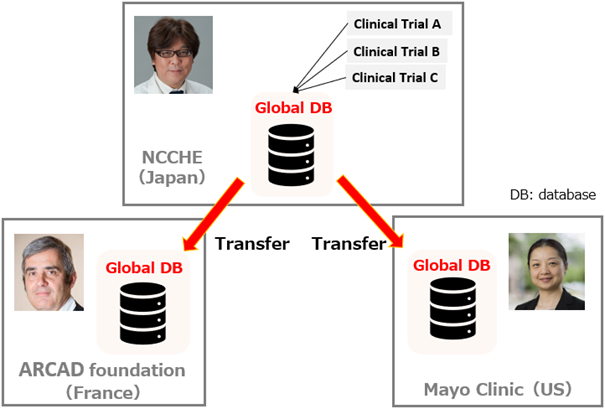
Comment from Dr. Takayuki Yoshino (Deputy Director/Head, Department of Gastrointestinal Oncology, National Cancer Center Hospital East):
We are pleased that the ARCAD database has been extended to gastric cancer, and we hope that the ARCAD database will help streamline the development of new cancer drugs, further our understanding of the pathogenesis of the disease, and generate evidence so that more patients can receive appropriate cancer treatment as quickly as possible. The project strives to grow into a large and comprehensive database through further expansion of cancer types.Comment from Professor Thierry André (The ARCAD Foundation):
The ARCAD Foundation and myself are delighted that Pr Takayuki Yoshino is the leader of this initiative with the team of the National Cancer Center Hospital East in Japan, and we will do everything in our power to help him succeed.We are confident that he will bring this project to successful works, and we encourage and support him in its development.
Comment from Professor Qian Shi (Director of Biostatistics Shared Resource, Mayo Clinic Comprehensive Cancer Center):
The Mayo SHARE team and I are honored to collaborate with Professor Takayuki Yoshino as he leads this important initiative alongside the exceptional team at the National Cancer Center Hospital East in Japan. Together, we are committed to working closely to ensure the success of ARCAD-Gastric. By contributing our strong statistical and data management expertise, we are confident in his ability to guide this endeavor to an outstanding outcome, and we look forward to supporting and collaborating with him throughout its development.Projections
Based on the experience gained from the ARCAD Database Project in colorectal cancer since the 2000s, the project aims to establish a global database of gastric cancer clinical trials. The database will be used for various analyses to generate evidence on a global scale, reduce and streamline the cost of research and development of pharmaceuticals, and ultimately improve the patient's benefit by reducing the cost and efficiency of patient benefit.
Overview of the institution
National Cancer Center Japan (https://www.ncc.go.jp/en/index.html)
The National Cancer Center was founded in 1962 as a national institution and has led cutting-edge research as a national core institution in cancer research and cancer treatment. The National Cancer Center Hospital East was established in 1992 and is one of the top cancer hospitals in Japan, with more than 11,000 new patients visiting the hospital annually. With a mission of providing world-class cancer care and creating new cancer treatment, the hospital has been selected by the government as a “Core Hospital for Clinical Research” and a “Core Hospital for Cancer Genome Medicine”. With the Center for Advanced Medical Research and Development, which is also affiliated with the hospital, it is a center for research and development based on an international network, promoting the development of advanced cancer drugs and medical devices and cutting-edge personalized treatment, including genomic medicine, and has achieved numerous results.
The ARCAD Foundation (https://www.fondationarcad.org/)(linked at external site)
The A.R.C.A.D. (Aide et Recherche en CAncérologie Digestive) Foundation, founded in 2006 by Aimery de Gramont and chaired since 2022 by Thierry André, is the only public research foundation in France dedicated to digestive cancer. The ARCAD Foundation focuses on patients, their caregivers and healthcare professionals in a variety of initiatives with three main objectives
-To support and promote clinical research and quality care
-Inform and support patients and their caregivers
-raising awareness among the public and health care professionals about the need for prevention and screening.
Mayo Clinic (https://www.mayoclinic.org/departments-centers/mayo-clinic-cancer-center)(linked at external site)
Mayo Clinic is a non-profit organization dedicated to clinical care, education, and research, providing professional and holistic care to all those in need of healing. Mayo Clinic's mission is to provide hope and contribute to the health and well-being of all patients by integrating clinical care, education, and research to provide the best care possible. Its main value is “The needs of the patient come first,” and it is considered to be top-ranked in quality above all other healthcare institutions. The Mayo Clinic of Minnesota has been recognized by U.S. News & World Report as one of the nation's best hospitals for 2024-2025.
The Mayo Clinic Comprehensive Cancer Center is the pinnacle of cancer treatment, research and education. Each year, more than 130,000 cancer patients from around the world visit the Mayo Clinic to receive an accurate diagnosis and treatment tailored to their specific needs and cancer type. For more than 50 years, the Mayo Clinic has focused on a team approach to finding new and better ways to prevent, stop, diagnose, and treat cancer, helping to transform the nation's cancer research efforts.
Terminology
*1 phase II and III trials
Clinical trials are largely divided into Phase I through Phase III. In Phase II, a relatively small number of patients will be studied to determine efficacy, safety, and use of the drug, including dosage and administration, when and at what time of day it is most effective, and which method has the fewest side effects. In Phase III, we will confirm the efficacy, safety, and use of the drug in many patients, based on the results of the Phase II study.
*2 PD-1/PD-L1 and MSI
PD-1/PD-L1:
A protein involved in the immune regulatory system, cancer cells produce PD-L1 on their cell surface to fend off attacks from immune cells (T cells). Immune checkpoint inhibitors are drugs that suppress PD-1 and PD-L1 so that T cells can attack cancer cells.
MSI (microsatellite instability):
A phenomenon in which the number of repetitions of repeating sequences (microsatellites) of one to several nucleotides is altered due to a decrease in MMR(Mismatch repair) function, which repairs errors that occur during DNA replication. When MMR function is defective, DNA errors are not repaired and can accumulate, resulting in cancer.
*3 ARCAD Asia Data Center (https://www.ncc.go.jp/jp/ncce/division/arcadasia/index.html)
“ARCAD Asia Steering Office and Data Center” has been established within the East Hospital, and joint research agreements for data provision have been concluded with various pharmaceutical companies to establish a data sharing system within the consortium. As of December 2024, data collection and integration has been completed for a total of 13 trials including 4,173 cases of colorectal cancer data between ARCAD Asia and data providers such as companies and academia. The data will be transferred to Mayo Clinic and integrated with European and U.S. trial data to establish the ARDAD-Global DB every year.
◆Members (as of December 2024):
Atsushi Ohtsu (Honorary Director National Cancer Center Hospital East); Yoshihiko Maehara (Director, Kyushu Central Hospital of the Mutual Aid Association of Public School Teachers); Takayuki Yoshino (Deputy Director/Head, Division of Drug and Diagnostic Development Promotion, National Cancer Center Hospital East); Eiji Oki (Associate Professor, Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University); Kentaro Yamazaki (Chief, Division of Gastrointestinal Oncology and Clinical Trial Coordination Unit, Shizuoka Cancer Center); Hideaki Bando (Chief, Department of Gastrointestinal Oncology/Chief, Division of Drug and Diagnostic Development Promotion, National Cancer Center Hospital East); Masanori Terashima (Deputy Director, Shizuoka Cancer Center); Kohei Shitara (Chief, Department of Gastrointestinal Oncology, National Cancer Center Hospital East); Yoshihiro Kakeji (Chief , Department of Esophago-Gastroenterological Surgery, Graduate School of Kobe University)
◆Reference
September 30, 2021 press release
Launch of “ARCAD Asia,” an international clinical trial data sharing project aiming to lead the world's R&D by leading the data sharing environment in the Asian region.
https://www.ncc.go.jp/jp/information/pr_release/2021/0930/index.html
August 4, 2022 press release
Approximately 43,000 individual patient data of clinical trials with colorectal cancer in Asia and the West shared ―ARCAD Database Project has established new international data-sharing framework promoting R&D in cancer medicine―
https://www.ncc.go.jp/en/information/press_release/2022/0804/index.html
Contact
On Project
National Cancer Center Hospital East
ARCAD Asia Management Office / Data Center
Dr. Toshihiro Misumi, Division of Drug and Diagnostic Development Promotion, Department of Data Science
Phone: +81-4-7130-0222
Email:tmisumi●east.ncc.go.jp
ARCAD foundation
Thierry André
4 Rue kléber, 92300, Levallois-Perret, France
Phone: + 33 6 61 77 07 08
E-mail: thierry.andre●aphp.fr
Mayo Clinic
Dr. Qian Shi, Professor of Biostatistics and Oncology
Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA;
Phone: +1 507-538-4340
E-mail: shi.qian2●mayo.edu
From the press and media
National Cancer Center Japan
Office of Public Relations, Strategic Planning Bureau (Kashiwa Campus)
E-mail: ncc-admin●ncc.go.jp
