Home > ARCADAsia > What is ARCAD Asia?
What is ARCAD Asia?
- International Clinical Trial Data Integration Project: ARCAD Asia is a data sharing project to collect and integrate data on past clinical trials and studies carried out primarily in Asia and promote the use of such data for drug research and development.
- With the cooperation of pharmaceutical companies and academia clinical research groups, ARCAD Asia collects past clinical trials and studies in a data center (ARCAD Asia Data Center) established in the National Cancer Center and builds a database.
- Utilizing and applying diverse past clinical trial/study results stored in the database enables integrated analyses that are impossible to perform in a single research project, facilitating a broad range of research approaches. In addition, it is expected to help streamline drug research and development activities, making it possible to provide better treatments to patients more quickly.
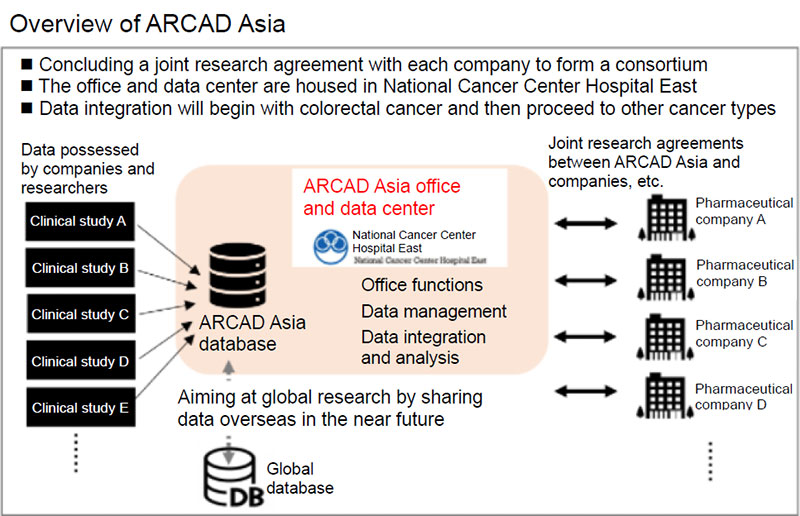
The ARCAD Asia Data Center is established in National Cancer Center Hospital East, a joint research agreement is made between the National Cancer Center and each pharmaceutical company or other organization, and a data sharing system is built within the consortium. Clinical trial/study data is provided by pharmaceutical companies and academia research groups under agreement and an integrated database is built in the data center. If a consortium member or academia wishes to use the integrated database, an Academia Group gives thorough consideration based on medical importance and appropriateness and then the ARCAD Asia Data Center performs analysis and provides the analysis results to the requester.
This project starts by sharing clinical trial and study data on unresectable, advanced and recurrent colorectal cancer cases (stage 4). Since stage 4 cancer is the area in which pharmaceutical companies prioritize drug research and development, the project is expected to be utilized also for developing new drugs. As many clinical development projects for stage 4 cancer are underway in Asia, especially in recent years, Japan aims to drive an Asian data sharing environment by working on infrastructure development ahead of others.
Significance and Necessity of Data Sharing
Data Sharing is an attempt to build a database of data on clinical studies and other research activities that individual companies possess by collecting such data into a single location with personal identification information removed so that it can be shared and utilized by academia and other third parties.
Normally valuable patient data collected to carry out clinical trials/studies is used to achieve results for those trials and studies and then retained by individual companies and other academia.
Meanwhile, since carrying out a clinical trial or study requires a great deal of effort and cooperation from patients as well as a long period of time and a large amount of money from the company, the idea of data sharing, or an approach to making the most of such valuable, dormant data, has been explored in recent years.
In Europe and the United States, data sharing infrastructures have already been built for more efficient drug research and development as well as for data utilization in various research projects, producing positive outcomes.
Project Schedule
The project will start with clinical trials/studies on unresectable colorectal cancer cases carried out primarily in Asia.
Afterwards it will cover more cancer types and coordinate with other Asian countries, thereby building an Asia-wide data center in Japan.
Note: Project progress will be announced on this website as needed.
ARCAD Asia Database Analyses Impact
- Development of surrogate endpoints (indicators for evaluating effect more easily that are used when it is difficult to evaluate effect objectively or in the period of a clinical trial)
- Development of a new disease stage classification system (reconstruction of intrinsic prognostic factors)
- Consideration of rare subjects such as elderly and young patients, etc.
Future prospects
ARCAD Asia will systematically evaluate regulatory science issues by creating an Asia-wide data sharing environment, building a database that integrates existing clinical trials/studies primarily on colorectal cancer with future clinical studies to be conducted, and performing various analyses using that database. Based on the results, we will offer proposals on endpoints of clinical studies and pharmaceutical approval processes so as to make drug research and development more cost-effective and efficient and maximize the benefit for patients.
In addition, we will utilize the infrastructure of this project to integrate our database with the ones in Europe and the United States in the future so as to build a global cancer clinical study database, allow for various analyses that use a large volume of data, and thereby promote the creation of evidence and development of treatments on a global scale.
