Home > Organization > Divisions and Independent Research Units > Division of Cancer Therapeutics > Research Highlights
Research Highlights

Our division focuses on identifying synthetic lethal targets for cancers with specific genetic vulnerabilities. Below are selected highlights of our breakthroughs in defining novel therapeutic strategies.
【Highlight 1: The Latest Breakthrough (2024)】
Project: Targeting Paralogous Dependencies in SMARCB1-Deficient Cancers
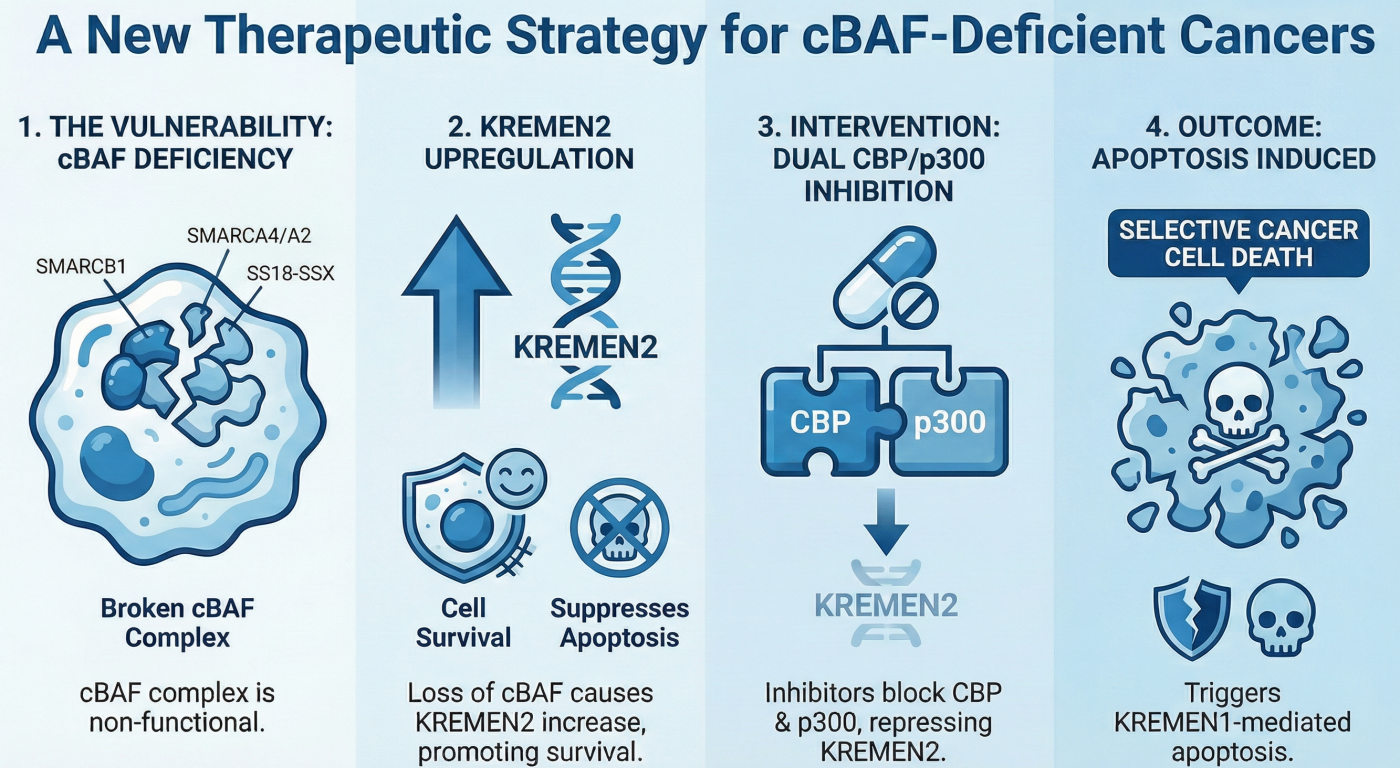
- The Challenge: SMARCB1-deficient cancers (e.g., malignant rhabdoid tumors, epithelioid sarcoma) are aggressive pediatric/AYA cancers with no effective molecular therapies.
- Our Discovery: We identified a "paralog dependency" between the histone acetyltransferases CBP and p300. While inhibiting one alone was insufficient, simultaneous inhibition of the CBP/p300 paralog pair induced complete synthetic lethality in SMARCB1-deficient cells.
- Mechanism: This dual inhibition prevents the "de-repression" of KREMEN2, a gene critical for cell survival in this context.
- Publication: Nature Communications, 2024
Sasaki M, Kato D, Murakami K, Yoshida H, Takase S, Otsubo T, Ogiwara H.
Targeting dependency on a paralog pair of CBP/p300 against de-repression of KREMEN2 in SMARCB1-deficient cancers.
Nat Commun. 2024 15(1):4770.
[ Read Full Paper ]
【Highlight 2: Metabolic Targeting (2019)】
Project: Exploiting Metabolic Vulnerabilities in ARID1A-Mutated Cancers
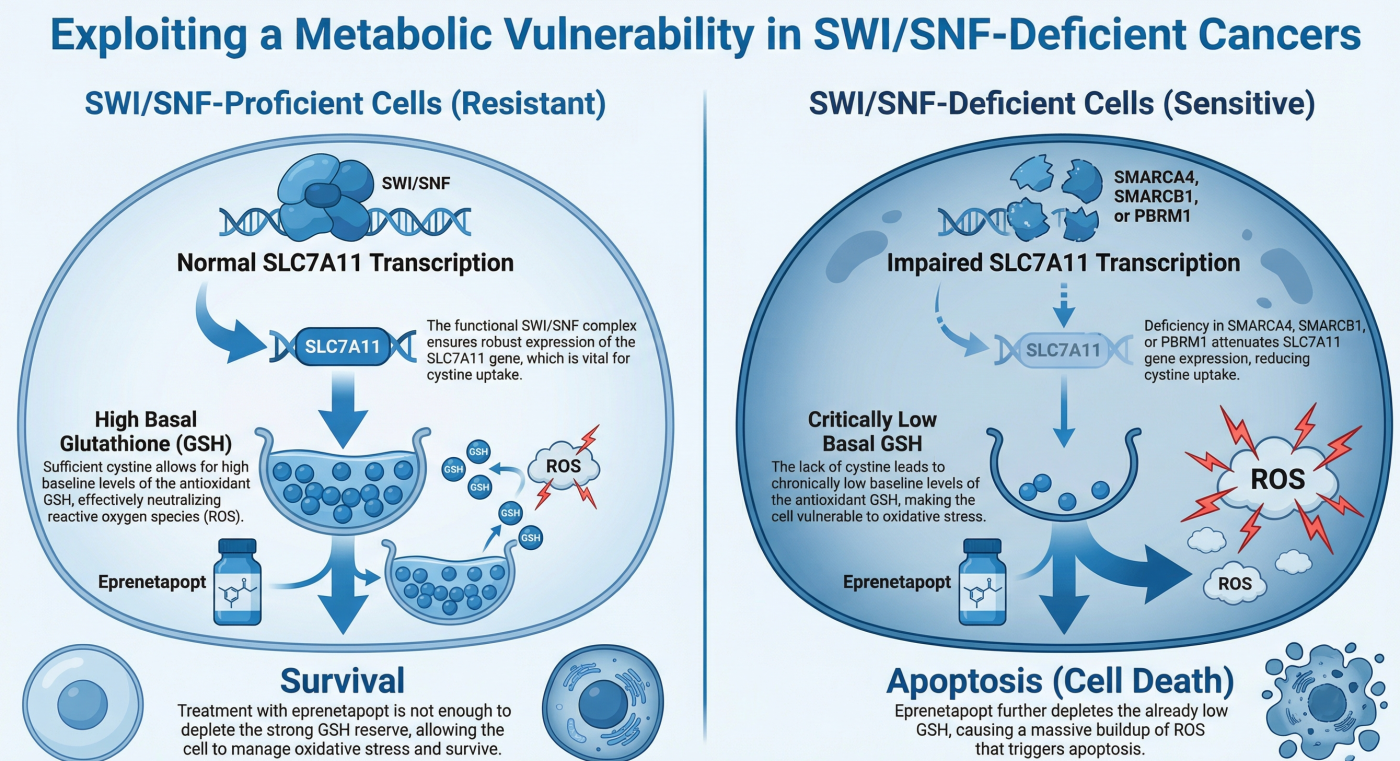
- The Challenge: ARID1A mutations are frequent in ovarian clear cell carcinoma and other refractory cancers, yet they lack direct drug targets.
- Our Discovery: We discovered that ARID1A-deficient cancer cells have a critical vulnerability in Glutathione (GSH) metabolism. These cells specifically depend on the metabolic enzyme GCLC for survival due to compromised SLC7A11 expression.
- Clinical Potential: GCLC inhibitors effectively eliminated ARID1A-deficient tumors in vivo, suggesting a novel metabolic therapeutic strategy.
- Publication: Cancer Cell, 2019
Ogiwara H., Takahashi K., Sasaki M., Kuroda T., Yoshida H., Watanabe R., Maruyama A., Makinoshima H., Chiwaki F., Sasaki H., Kato T., Okamoto A., Kohno T.
Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers.
Cancer Cell. 35:177-190.e8. 2019
[ Read Full Paper ]
【Highlight 3: Synthetic Lethality Foundation (2016)】
Project: CBP/p300 Synthetic Lethality in CBP-Deficient Cancers
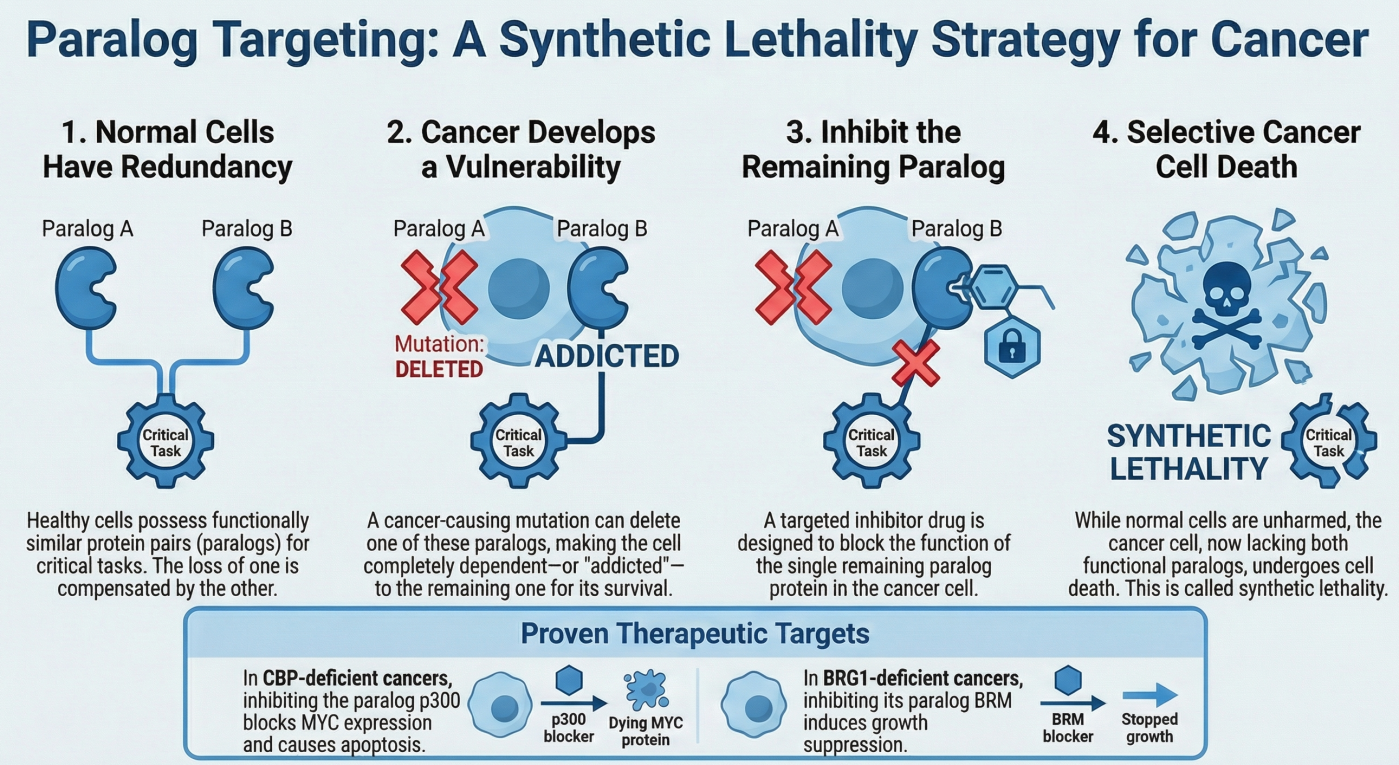
- The Discovery: We revealed that cancers with CBP gene loss are fundamentally dependent on its paralog, p300.
- Mechanism: Inhibition of p300 in CBP-deficient cells abrogates MYC expression, leading to apoptotic cell death. This established the concept that targeting paralogous HATs (Histone Acetyltransferases) is a viable strategy for chromatin-deficient cancers.
- Publication: Cancer Discovery, 2016
Ogiwara H., Sasaki M., Mitachi T., Oike T., Higuchi S., Tominaga Y., Kohno T.
Targeting p300 addiction in CBP-deficient cancers causes synthetic lethality via apoptotic cell death due to abrogation of MYC expression
Cancer Discov. 6(4):430-445. 2016
[ Read Full Paper ]
【Highlight 4: Early Pioneering Work (2013)】
Project: Targeting BRG1 Deficiency
- The Discovery: One of our foundational studies demonstrating that BRG1-deficient cancer cells are dependent on the ATPase activity of BRM (SMARCA2). This work pioneered the strategy of targeting residual SWI/SNF complex members in deficient cancers.
- Publication: Cancer Research, 2013
Oike T., Ogiwara H., Tominaga Y., Ito K., Ando O., Tsuta K., Mizukami T., Shimada Y., Isomura H., Komachi M., Kohno T.
A synthetic lethality-based strategy to treat cancers harboring a genetic deficiency in the chromatin remodeling factor BRG1.
Cancer Res. 73:5508-5518. 2013
[ Read Full Paper ]
Are you interested in our drug discovery seeds? → [ Contact for Collaboration ]

