Home > Organization > Division of Developmental Therapeutics(Kashiwa) > Research Summary
Research Summary
Development of antibody–drug conjugates (ADCs) and radioimmunotherapy (RIT) as armed therapeutic antibodies
Although clinical applications of antibody therapeutics and next-generation antibody therapeutics centered on ADCs have progressed, they have not been applied to refractory cancers such as pancreatic and diffuse gastric cancers. We reported that drug delivery could be disturbed by dense stroma in these tissues (i.e., the tumor stromal barrier). A novel method or technology for antibody delivery is required to overcome this type of barrier and carry to the tumor cells.
The antibody affinity constant is calculated from the rate constants of kinetic association (kon) and dissociation (koff). We found that adjustment of koff could improve antibody penetration within tumor tissue. Based on this finding, we will continue to pursue antibody engineering–based efforts to develop an antibody capable of deeply penetrating stroma-rich pancreatic or diffuse gastric tumor tissue.
Molecular imaging is very useful for visually evaluating antibody delivery. In addition, refractory or remnant tumor cells themselves are likely to be resistant to general chemotherapy. Alpha emitters with high-energy and linear-energy transfer, which exert greater cytotoxicity, could be used for the treatment of chemoresistant-refractory cancer. Hence, we pursue pharmaceutical research and development of ADCs and RIT in collaboration with professionals in various research fields.

【Reference】
-
Yasunaga M, Manabe S, Matsumura Y. CAST therapy. In Matsumura, Y. and Tarin, D. (Eds.), Cancer Drug Delivery Systems Based on the Tumor Microenvironment. Springer. 269-288, 2019.
-
Koga Y, Tsumura R, Matsumura Y. Preclinical Studies of ADC Therapy for Solid Tumors. Cancer Drug Delivery Systems Based on the Tumor Microenvironment. Springer. 125-154, 2019.
-
Tsumura R, Manabe S, Takashima H, Koga Y, Yasunaga M, Matsumura Y. Evaluation of the antitumor mechanism of antibody-drug conjugates against tissue factor in stroma-rich allograft models. Cancer Sci. 2019 110(10): 3296-3305.
-
Fuchigami H, Manabe S, Yasunaga M, Matsumura Y. Chemotherapy payload of anti-insoluble fibrin antibody-drug conjugate is released specifically upon binding to fibrin. Sci Rep. 8, 14211-14219. 2018.
-
Tsumura R, Manabe S, Takashima H, Koga Y, Yasunaga M, Matsumura Y. Influence of the dissociation rate constant on the intra-tumor distribution of antibody-drug conjugate against tissue factor. J Control Release. 284, 49-56. 2018.
-
Tsumura R, Sato R, Furuya F, Koga Y, Yamamoto Y, Fujiwara Y, Yasunaga M, Matsumura Y. Feasibility study of the Fab fragment of a monoclonal antibody against tissue factor as a diagnostic tool. Int J Oncol. 47, 2107-14. 2015.
-
Koga Y, Manabe S, Aihara Y, Sato R, Tsumura R, Iwafuji H, Furuya F, Fuchigami H, Fujiwara Y, Hisada Y, Yamamoto Y, Yasunaga M, Matsumura Y. Antitumor effect of antitissue factor antibody-MMAE conjugate in human pancreatic tumor xenografts. Int J Cancer. 37, 1457-66. 2015.
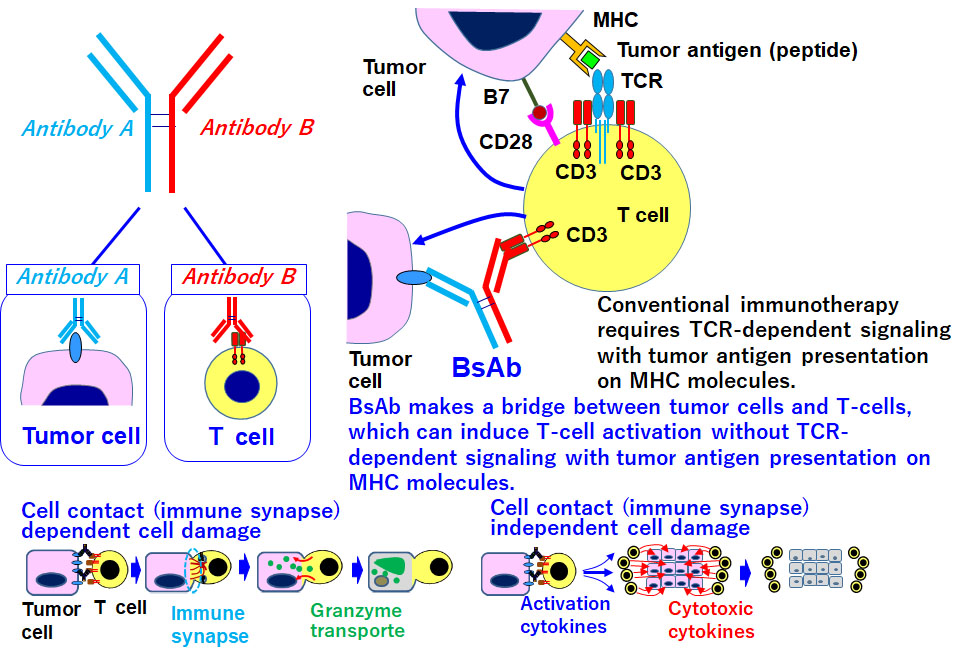
Development of methods for T cell regulation to facilitate clinical application of bispecific antibody
Over 50 clinical trials involving BsAbs are ongoing worldwide. However, this technology has been applied to hematopoietic malignancies, but not to solid tumors. The antitumor efficacy of ADCs or RIT depends on the cytotoxic agent or radioisotope used as the payload, whereas the efficacy of a BsAb could be determined by activation of T cells redirected to the tumor. Therefore, the efficacy depends on the activation of host T cells as well as the BsAb itself. This is why pharmaceutical research and development of BsAbs themselves are limited, and an approach for regulating T cells in the body is urgently required. T cell deserts and exhaustion are major obstacles and must be overcome in order to achieve stable antitumor efficacy in clinics. Accordingly, we will seek to develop new technology for the strict temporal and spatial regulation of host T cells (T cells as DDS drugs) through close cooperation with researchers in fields such as DDS, molecular imaging, protein engineering, gene engineering, and cell biology.
【Reference】
-
Kamakura D, Asano R, Kawai H, Yasunaga M. Mechanism of action of a T cell-dependent bispecific antibody as a breakthrough immunotherapy against refractory colorectal cancer with an oncogenic mutation. Cancer Immunol Immunother. 2020.
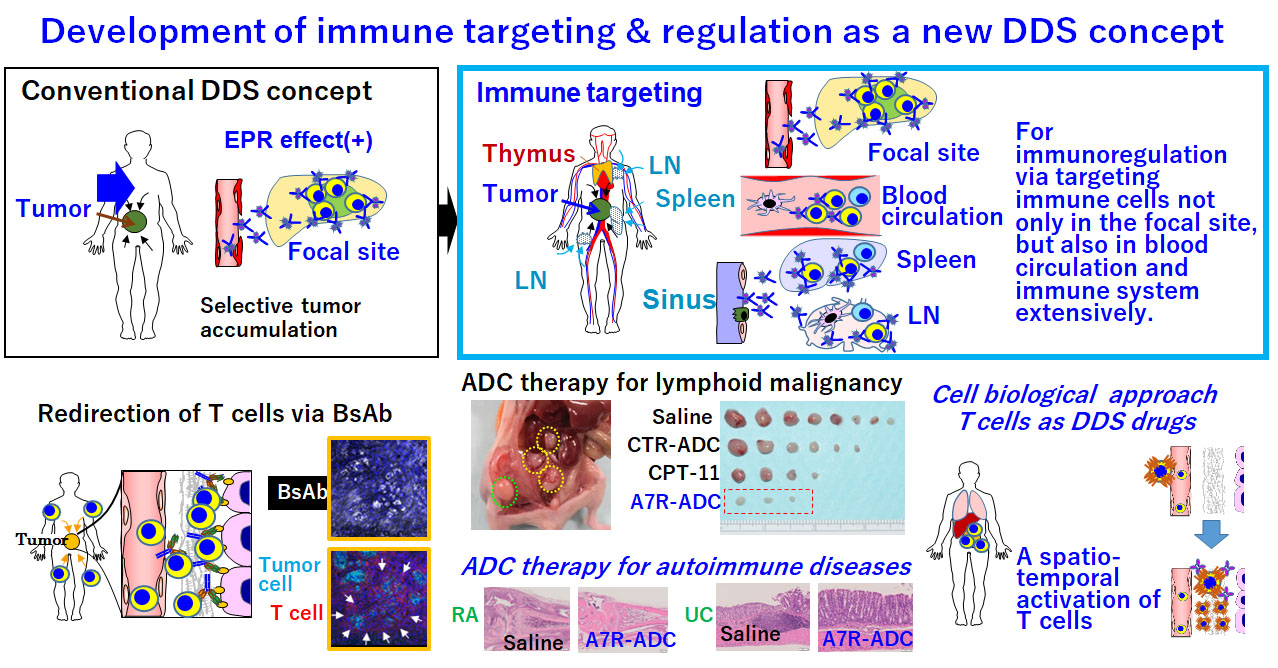
Development of immune targeting and regulation as a new DDS concept
Most DDS drugs have been developed according to the conventional DDS concept, focusing primarily on the EPR effect, which allows high–molecular weight DDS drugs to accumulate in tumors. On the other hand, we will continue to exploit immune targeting as a new DDS concept. For immune targeting and regulation via targeting of immune cells extensively not only in tumor or pathological focal site but also in blood circulation and immune system such as thymus, bone marrow, lymph node, and spleen. We consider that BsAb-dependent T cell redirection should be categorized as a form of immune targeting and regulation. Anti–IL-7R ADCs may be useful for treating leukemia or autoimmune disease by overcoming steroid resistance, attenuating lymphocyte-homing activity, or interfering with disease-specific signaling molecules. Furthermore, in an overlapping BsAb project, we will develop a novel T cell regulation technology for controlling the spatiotemporal activation of T cells in the body; we refer to this concept as “T cells as DDS drugs”.
【Reference】
-
Yasunaga M. Antibody therapeutics and immunoregulation in cancer and autoimmune disease. Semin Cancer Biol. Semin Cancer Biol. 64, 1-12. 2020.
-
Yasunaga M, Manabe S, Matsumura Y. Immunoregulation by IL-7R-targeting antibody-drug conjugates: overcoming steroid-resistance in cancer and autoimmune disease. Sci Rep. 7, 10735. 2017.
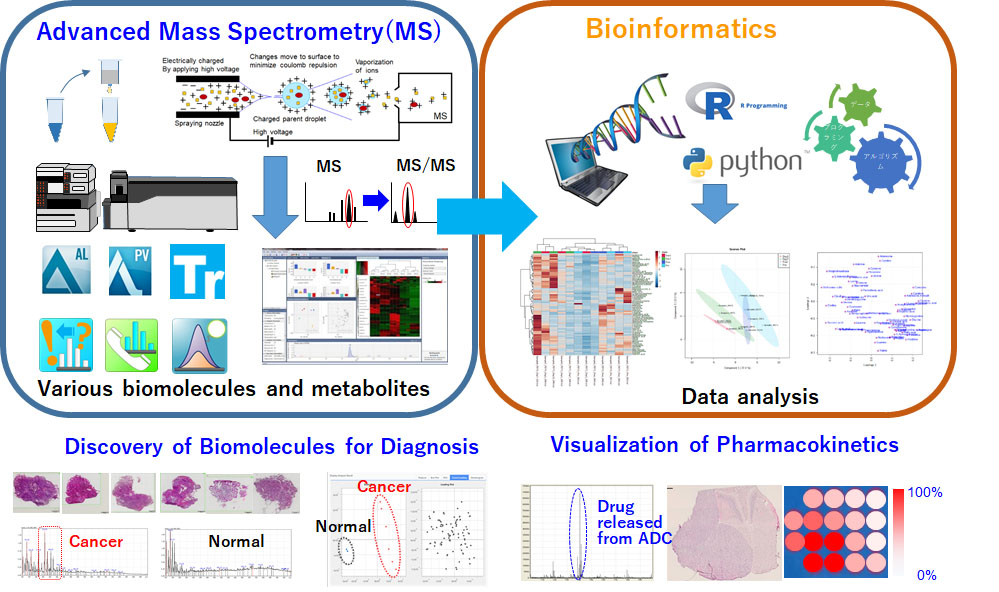
Advanced mass spectrometry and bioinformatics systems for pharmaceutical research and development
Using mass spectrometry–based proteomics and metabolomics along with bioinformatics, we will continue to discover target molecules for the development of therapeutic antibodies. Moreover, mass spectrometry studies allow us to visualize payloads released from ADCs and observe the signature of drug-induced cell injury by monitoring changes in the biomolecules and metabolites influenced by them. Such approaches would be helpful for elucidating the bystander effect and immunogenic cell death (ICD), which lie at the cutting edge of ADC research.
In addition, the immaturity of methods and databases is a major issue in mass spectrometry informatics. It is very difficult to identify biomolecules or metabolites in tissue samples even if their molecular size (mass-to-charge ratio, m/z) has been determined, because 90% molecules are currently un-annotated. Therefore, we will continue to introduce in situ–level mass spectrometry analysis and the latest computer science–based data analysis technologies, including artificial intelligence (AI). These advanced mass spectrometry and bioinformatics systems allow us to evaluate the mechanism of action (MOA), pharmacokinetics (PK), and pharmacodynamics (PD) of new drugs. They are also useful for discovering new biomolecules as therapeutic targets.
【Reference】
-
Yasunaga M, Manabe S, Furuta M, Ogata K, Koga Y, Takashima H, Nishida T, Matsumura Y. Mass spectrometry imaging for early discovery and development of cancer drugs. AIMS Md Sci. 5, 162-180. 2018.
-
Fujiwara Y, Furuta M, Manabe S, Koga Y, Yasunaga M, Matsumura Y. Imaging mass spectrometry for the precise design of antibody-drug conjugates. Sci Rep. 6, 24954. 2016.
-
Yasunaga M, Furuta M, Ogata K, Koga Y, Yamamoto Y, Takigahira M,Matsumura Y. The significance of microscopic mass spectrometry with high resolution in the visualisation of drug distribution. Sci Rep. 3, 3050. 2013.
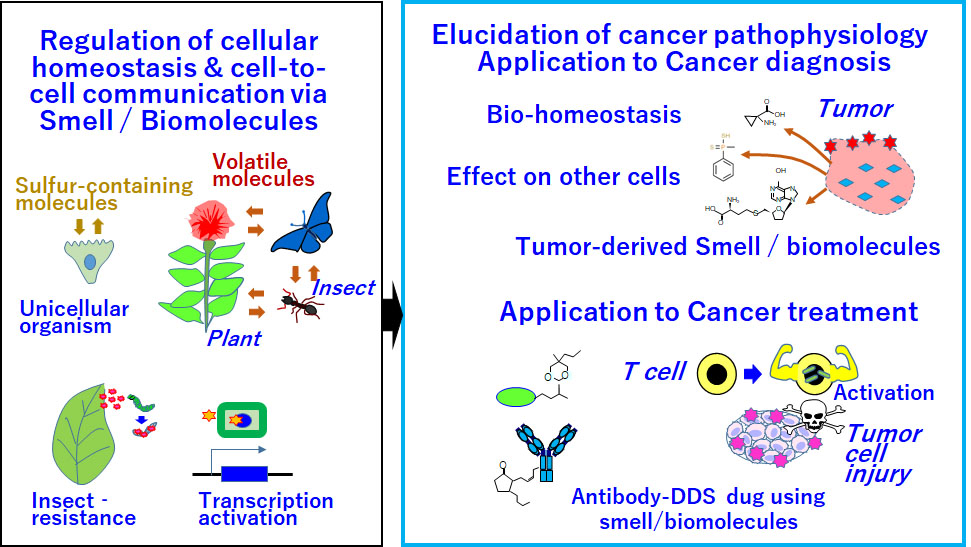
Development of new oncology methods targeting smell and biomolecules for application to cancer diagnosis and treatment
Out-of-the-box thinking is required to develop medications for refractory cancers such as pancreatic and diffuse gastric cancers. Cancer-derived smell is a challenging research topic related to cancer diagnosis and treatment. Technologies based on such smells might open a new era of oncology.
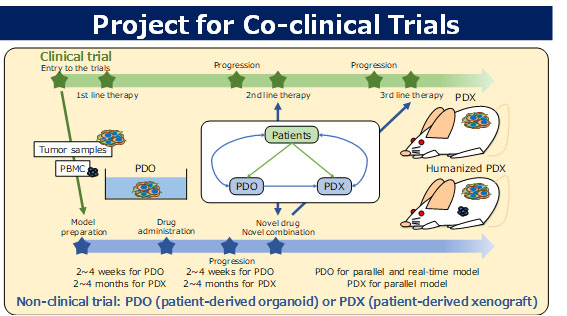
Co-clinical trial project
We are developing various drugs based on DDS technologies; however, discordance between the results of non-clinical research and clinical trials is a major issue in the development of anticancer drugs. This is because non-clinical models are not able to accurately reproduce all aspects of the human cancer microenvironment, including the interactions between cancer cells and the stroma. In the co-clinical trial project, we will clarify the characteristics of various non-clinical models such as two-dimensional cell cultures, three-dimensional cell cultures (spheroids and organoids), cell line–derived xenografts (CDX), and patient-derived xenografts (PDX), and construct a suitable non-clinical drug evaluation system for the development of DDS-based drugs.
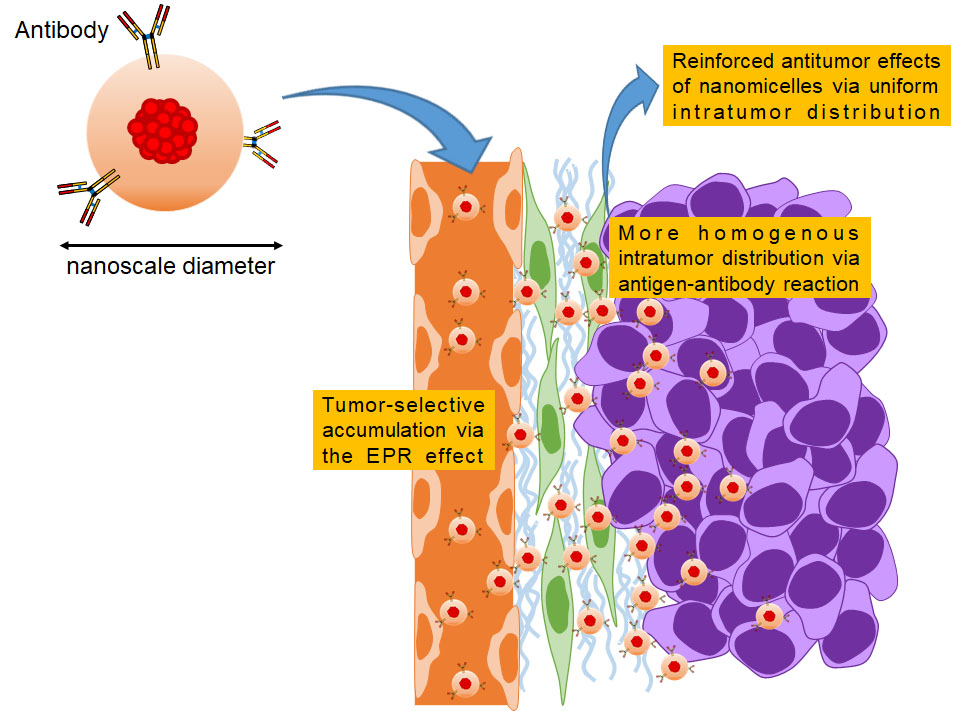
DDSs based on nanotechnology
DDSs based on nanotechnology are among the most promising strategies for tumor-selective accumulation of therapeutics. We are currently focusing on developing polymeric micelles containing antitumor agents. It has been preclinically and clinically proven that micellar formulations are safer than conventional low–molecular weight drugs due to reduced distribution to normal organs via the EPR effect. To develop clinically useful micellar formulations, it is important to increase antitumor activities without affecting safety. We reported that when antibodies were attached to their outer shells, micelles could exert a reinforced antitumor effect without worsening toxicity. Based on our pharmacokinetic investigations, we will reveal the MOAs of micelles containing therapeutic agents, leading to the development of approved formulations in the future.
【Reference】
-
Takashima H, Koga Y, Tsumura R, Yasunaga M, Tsuchiya M, Inoue T, Negishi E, Harada M, Yoshida S, Matsumura Y. Reinforcement of antitumor effect of micelles containing anticancer drugs by binding of an anti-tissue factor antibody without direct cytocidal effects. J Control Release. 323, 138-150. 2020.
-
Takashima H, Tsuji AB, Saga T, Yasunaga M, Koga Y, Kuroda JI, Yano S, Kuratsu JI, Matsumura Y. Molecular imaging using an anti-human tissue factor monoclonal antibody in an orthotopic glioma xenograft model. Sci Rep. 7, 12341. 2017.
Links.
- Laboratory of Cancer Biology, Department Integrated Biosciences, The university of Tokyo (Link to External Site.)
